-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2020; 9(1): 11-16
doi:10.5923/j.ijvmb.20200901.03

Clinical Insights into the Pandemic of COVID-19: An Updated Review till the End of March 2020
Mosaad Morsy, Ahmed Elgebaly, Hussien Ahmed, Reham Elgarhy, Fady Adel, Mahmoud Shehata
RAY, Contract Research Organization, Giza, Egypt
Correspondence to: Hussien Ahmed, RAY, Contract Research Organization, Giza, Egypt.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Coronavirus disease 2019 (COVID‐19) has emerged as a global public health emergency, which is characterized by high infection rate and fatal course, especially in vulnerable groups. COVID-19 is a worldwide pandemic that is caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). To date, the COVID-19 has affected almost 129 countries around the world, and there are 693,224 confirmed cases by the end of March 2020. SARS-COV-2 is contagious, single-stranded RNA, the virus that is mainly presented in different animal species. Patients with SARS‐CoV‐2 infection can present with a variety of presentations ranging from a mild form of flu-like symptoms to fatal pneumonia and respiratory failure; most common presentations of COVID-19 include fever, cough, fatigue, and to lesser extent diarrhea and vomiting. To date, there is no curative treatment of COVID-19, and the treatments of patients with SARS-CoV-2 infection are mainly symptomatic treatment. The most commonly used drugs include empirical antibiotics, antiviral therapy (oseltamivir), and systemic corticosteroids [8]. In addition, potential therapies for COVID-19 are currently studied, such as neuraminidase inhibitors, remdesivir, chloroquine, and hydroxychloroquine (HCQ) in patients with SARS-CoV-2-related pneumonia. In the present review, we aim to present updated clinical insights about the current data regarding the COVID-19 pandemic.
Keywords: COVID-19, Pandemic, Literature review
Cite this paper: Mosaad Morsy, Ahmed Elgebaly, Hussien Ahmed, Reham Elgarhy, Fady Adel, Mahmoud Shehata, Clinical Insights into the Pandemic of COVID-19: An Updated Review till the End of March 2020, International Journal of Virology and Molecular Biology, Vol. 9 No. 1, 2020, pp. 11-16. doi: 10.5923/j.ijvmb.20200901.03.
Article Outline
- The Since the appearance of its first case in early December in the Wuhan area in China's Hubei province, coronavirus disease 2019 (COVID‐19) has emerged as a global public health emergency, which is characterized by high infection rate and fatal course, especially in vulnerable groups [1]. COVID-19 is a worldwide pandemic, as declared by World Health Organization (WHO) on March 11, 2020, that is caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) [2]. To date, the COVID-19 has affected almost 129 countries around the world, and there are 693,224 confirmed cases by the end of March 2020 [3]. Although the disease initially spread in China, its epidemiology has changed dramatically in which both the United States (US) and Italy are harboring most of the confirmed cases. SARS-COV-2 is contagious, single-stranded RNA, the virus that is mainly presented in different animal species. Initially, the animal-to-human transmission was presumed as the primary mechanism; nonetheless, the widespread of confirmed cases has reflected a strong human-to-human transmission, mainly via respiratory droplets [4].Patients with SARS‐CoV‐2 infection can present with a variety of presentations ranging from a mild form of flu-like symptoms to fatal pneumonia and respiratory failure [5]; most common presentations of COVID-19 include fever, cough, fatigue, and to lesser extent diarrhea and vomiting [6]. In patients with comorbidities and elderly group, a variety of complications may present, such as acute respiratory distress syndrome, acute heart injury, and secondary infection [7].To date, there is no curative treatment of COVID-19, and the treatments of patients with SARS-CoV-2 infection are mainly symptomatic treatment. The most commonly used drugs include empirical antibiotics, antiviral therapy (oseltamivir), and systemic corticosteroids [8]. In addition, potential therapies for COVID-19 are currently studied, such as neuraminidase inhibitors, remdesivir, chloroquine, and hydroxychloroquine (HCQ) in patients with SARS-CoV-2-related pneumonia [9]. In the present review, we aim to present updated clinical insights about the current data regarding the COVID-19 pandemic.
1. Review Development
- We conducted a comprehensive literature search on PubMed and Clinicaltrial.gov from their inception until March 31, 2020, to review all available data about the epidemiology, pathogenesis, presentation, prognosis, diagnosis, and management of COVID-19.
2. Etiology and Pathogenesis
- SARS-COV-2 is a member of the coronaviruses family, which are a group of single-strand RNA viruses with a crown-like appearance under light microscope. There are four human coronaviruses that mainly cause mild respiratory symptoms. Before the emergence of SARS-COV-2, two outbreaks of zoonotic coronavirus disease have occurred in the past two decades leading to SARS and the Middle East respiratory syndrome (MERS) [10]. In December 2019, a growing number of cases with serve pneumonia presented to Wuhan local hospitals, with common exposure to the Huanan wholesale seafood market. Later on, SARS‐CoV‐2 was discovered as the causative agent; it was found that SARS‐CoV‐2 had >95% homology with the bat coronavirus and > 70% similarity with the SARS- CoV [11]. Thus, the bats-to-human transmission is the most widely accepted initial event of COVID-19 outbreak. Nevertheless, human-to-human transmission is established with an exponential increase in the number of infected cases [12].Human-to-human transmission is believed to occur via respiratory droplets with an incubation period ranging from 3-14 days. In addition, the current data suggest that this novel epidemic doubled about every seven days, whereas the basic reproduction number (R0 - R naught) is 2.2 [13]. The current evidence suggests that the tramsimission of Infection can occur by both symptomatic and asymptomatic patients [14]. There is controversy regarding the duration in which the virus is still viable on the surfaces.Although the exact pathogenesis of COVID-19 is still largely unknown, the virulence mechanism of SARS-COV-2 is thought to depend on its non-structural and structural proteins. The angiotensin‐converting enzyme 2 (ACE2) is the receptor of SARS-COV-2 within the body, which predominately expressed in alveolar cells type II [15]. Once the virus binds with its receptor, the ACE2 expression is markedly elevated, leading to alveolar cell damage, with subsequent immune and inflammatory reactions within the body. Previous reports showed that patients with COVID-19 showed pulmonary edema and important proteinaceous exudates as large protein globules [16]. In severe form, a 'cytokine storm' can be initiated by the release of interleukin-6 (IL-6) and other pro-inflammatory cytokines leading to wide disturbance in thermoregulation, lymphocytes, central nervous system functions, and eventually death [12]. Cardiac involvement, such as acute myopericarditis and microthrombi of coronary arteries, was reported in patients with COVID-19 [17].
3. Epidemiology of COVID-19
- As mentioned before, the first outbreak of COVID-19 occurred in Wuhan in December 2019. The disease is highly contagious with a notable high reproduction number; for example, it was reported that the number of confirmed cases in China by February 2020 was1879 times of that in January 2020 (see Figure 1) [18]. According to March 30, 2020, report by WHO, the total number of confirmed cases was 693,224 worldwide. The vast majority of cases are concentered within the USA, European countries, and the Western Pacific region [3]. On 31 March 2020, the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University reported that there is a total of 777,291 confirmed cases, with the highest incidence in the USA, followed by Italy, Spain, and China (Figure 2) [19]. In addition, recent reports suggested that the actual number of infected cases is higher than reported as a considerable proportion of cases are asymptomatic. It is unclear why African countries have a low incidence rate of COVID-19; however, the authors suggested that high temperatures may play a role in limiting the spread of infection [20].
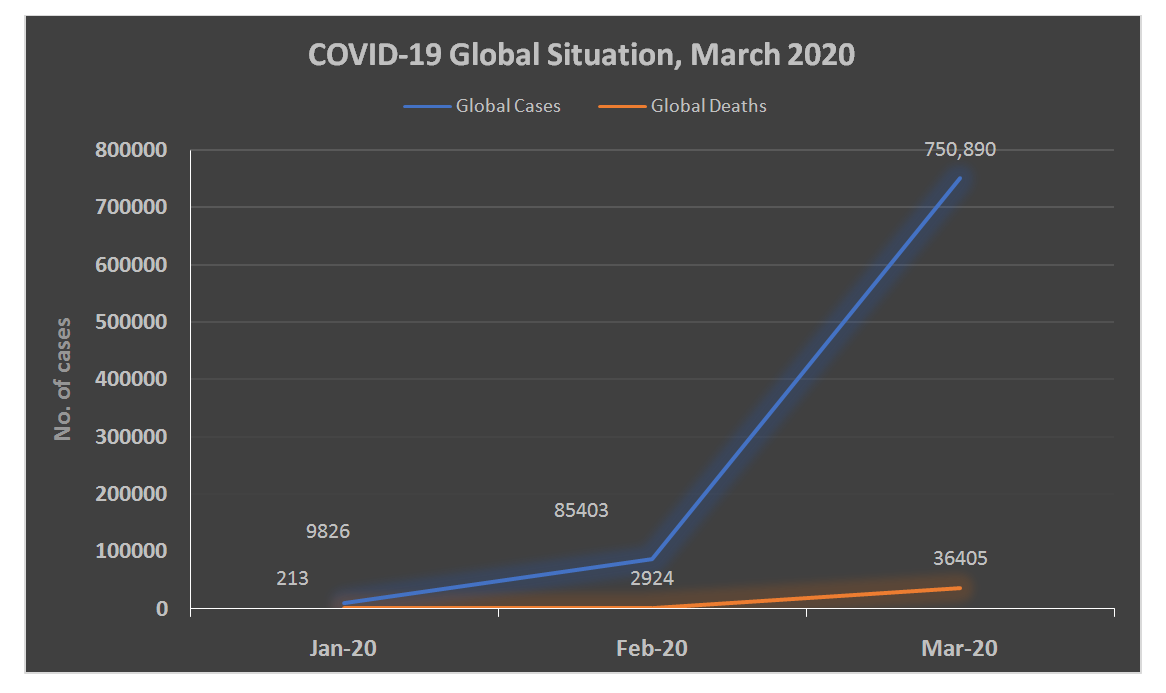 | Figure 1. COVID-19 Global Situation from January to March 2020 |
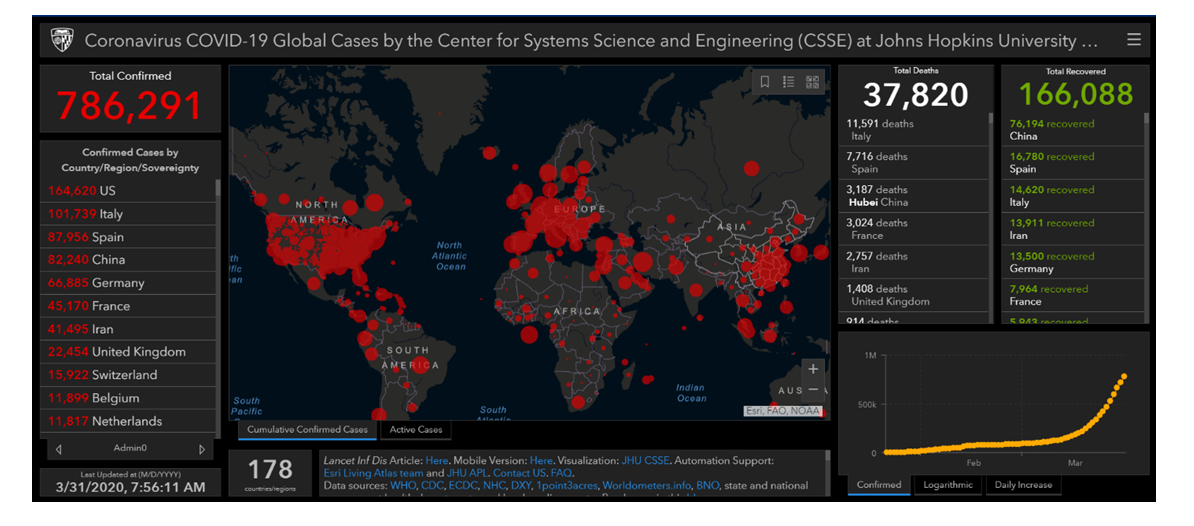 | Figure 2. Coronavirus COVID-19 Global Cases at the End of March 2020. Obtained from CSSE at Johns Hopkins University |
4. Clinical Presentation of COVID-19 and Prognosis
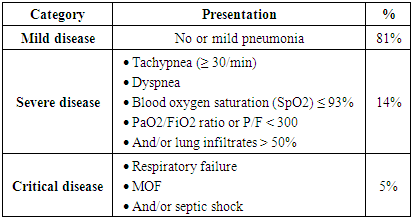
- The presentation of COVID-19 is highly variable and can range from asymptomatic/mild flu-like symptoms to fatal pneumonia, respiratory failure, and multiple organ failure. Large epidemiological studies from China highlighted that the incubation period can range from 3 to 14 days [4]. The most commonly reported features of COVID-19 are high fever, dry cough, dyspnea, headache, fatigue, myalgia, and to a lesser extent, nausea and vomiting [7]. Conjunctivitis was also reported as an early sign of infection. In vulnerable groups of patients (such as elderly and patients with immunocompromised conditions), the disease can progress to typical pneumonia, SARS, multiple organ failure, and eventually death [7].According to the Chinese CDC, the COVID-19 can be categorized into three groups according to the severity of the presentation (Table 1) [21].
|
5. Diagnosis of COVID-19
- Patients with SARS-COV-2 infection usually exhibit leucopenia and lymphopenia in complete blood count [23]. In addition, some patients may show elevated liver enzymes (alanine aminotransferase or aspartate aminotransferase), lactate dehydrogenase, and/or creatine kinase. An elevation in acute phase reactants and D-dimer can be present as well [12]. Previous reports also demonstrated a higher level of pro-inflammatory cytokines in patients with COVID-19 than healthy population, with prominent elevation in ICU patients [12].On chest computed tomography (CT), diverse and rapidly-progressing findings are usually present. Classic findings of patchy ground‐glass opacities (CGO) and patchy consolidation are usually located within the middle and outer zone of the lung; at least two lobes of the lung are affected in most cases. After improvement, lines of fibrous stripe may be present [24]. According to Pana and colleagues [25], four distinct stages of COVID-19 related changes in chest CT can be identified. The CGO is mainly confined to lower lobes, in one or both sides, in early stages. As the disease progress, diffuse CGO can be indemnified in both sides with consolidation in more than two lobes. These findings become more prominent in the peak stage. Finally, the absorption stage is characterized by gradual resolution of consolidation with persistent diffuse CGO.A combination of recent contact with confirmed cases, typical symptoms, and radiological findings are highly suggestive of SARS-COV-2 infection; however, the diagnosis is based on the detection of causative viruses by real time polymerase chain reaction (RT‐PCR) of respiratory swap [26]. Although RT-PCR is currently used as the primary diagnostic tool for suspected cases, the RT-PCR is limited by its low diagnostic accuracy (positive rate equal almost 60%); thus, the results of RT-PCR should be interpreted cautiously, and the test should be repeated again in negative cases [27]. Notably, a very recent report demonstrated that the chest CT was superior to RT-PCR in terms of detection of COVID-19, which suggests that chest CT can be used as a reliable method for diagnosis of COVID-19 [20].
6. Prevention and Treatment
6.1. Prevention
- To date, there is no approved vaccine for SARS-COV-2 infection; thus, the current recommended preventive strategy relies largely on general, non-specific measures. Nonetheless, factors like long incubation period, non-specific presentations, high rate of transmission from asymptomatic cases, and the possibility of transmission even after clinical recovery make the prevention of COVID-19 challenging [28].Home isolation is recommended in confirmed cases with no or mild symptoms; the home isolation should be applied to close contacts of confirmed cases as well. Cases should be isolated in a well-ventilated room with proper reach to sunlight. In addition, patients and caregivers should be instructed to wear surgical masks and practice proper hygiene measures. Patients with more advanced stages of the disease should be isolated within specialized hospitals (in separate or cohorted rooms). Regular decontamination by sodium hypochlorite should be done for surfaces and equipment. Unlike other flu pandemics, two consecutive negative molecular tests should be obtained before discharge [8].In order to limit disease transmission, many public health organizations recommend social dissociation and avoidance of crowded areas as possible. Travel restriction to areas with a high number of confirmed cases is recommended as well. People should be asked to practice cough hygiene by coughing in sleeve/ tissue rather than hands and practice hand hygiene frequently every 15–20 min. Although initial evidence did not support the use of surgical masks by healthy people, the current recommendations by the Centers for Disease Control and Prevention (CDC) emphasize the importance of wearing surgical masks by both healthy people and patients with respiratory symptoms, especially in crowded places [29].Healthcare workers (HCWs) are at high risk of SARS-COV-2 transmission. In China, a total of 3300 HCWs were infected with SARS-COV-2 so far, while almost 20% of HCWs, who were in close contact with COVID-19 cases, were infected [30]. Therefore, preventive measures should be followed strictly by HCWs; the infection control measures recommended for HCWs are those for category A agents (cholera, plague) [28]. HCWs should be fully equipped with personal protective equipment (PPE), which consists of N95 respirators, protective suits, and goggles. All contacts, including HCWs, should be monitored for the development of symptoms of COVID-19 [28].As per the March 20, 2020, update of the DRAFT landscape of COVID-19 candidate vaccines by WHO, there are two candidate vaccines which entered the clinical evaluation (phase I trials); while 42 vaccines are still in the pre-clinical phase (https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf?ua=1). In the firs published in-vitro study, the microneedle array delivered recombinant coronavirus vaccines showed promising results, which need further confirmation by in-vivo trials [31].
6.2. Treatment
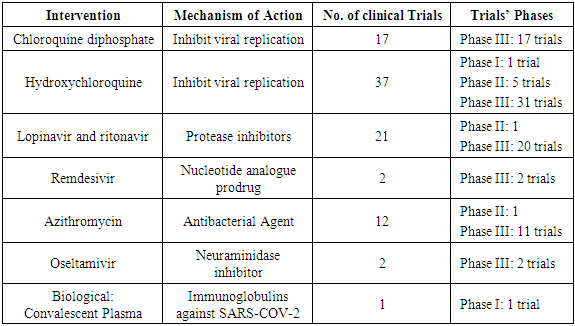
- At present, there are no specific antiviral drugs or vaccines against COVID-19 infection for potential therapy of humans. Thus, many national algorithms for the management of COVID-19 rely on symptomatic relief of the patients. Antiviral drugs, including nucleoside analogs and HIV-protease inhibitors, are used empirically in COVID-19 patients by many centers [32]. Initial reports from China demonstrated the use of oseltamivir, lopinavir, ritonavir, and ganciclovir for 3–14 days in the setting of COVID-19 [7]. More recent in-vitro studies showed that remdesivir and chloroquine were effective in reducing SARS-COV-2 viral replication [33]. An international trial “Solidarity trial” is planned by WHO to test the efficacy of at least four treatment options for the management of COVID-19.Furthermore, there are a number of other compounds that are in development. These include the clinical candidate EIDD-2801 compound that has shown high therapeutic potential against seasonal and pandemic influenza virus infections, and this represents another potential drug to be considered for the treatment of COVID-19 infection [34,35]. Convalescent plasma or immunoglobulin has been proposed as another promising agent for the fight against COVID-19 [36].As of March 31, 2020, there are 199 registered clinical trials on clinicaltrial.gov (Table 2).
|
7. Summary
- This review summarizes the current findings of SARS‐CoV‐2 along with the treatment for this severe CoV infection. The most common symptoms were addressed. Due to the only biopsy report, the pathological findings associated with SARS‐CoV‐2 infection have been limited. Autopsy is warranted and valuable for future research.The WHO issued a public health emergency of international concern on 30 January 2020. SARS‐CoV‐2 epidemic is becoming a global concern. At the moment, there is no vaccine and no specific treatment for COVID‐19. The best strategy to deal with SARS‐CoV‐2 epidemic includes controlling the sources of infection, protecting the susceptible people, and cutting off the transmission. The infected patients should be identified early by rapid and robust detection technologies, provided with optimized treatment in isolation timely. The close contact people should be quarantined with follow‐up. The healthy people should be aware of the severity of COVID‐19 and take measures to protect themselves, such as staying at home, limiting social contacts, and wearing protective mask in public. The authorities should encourage people to stay at home; discourage mass gathering; postpone or cancel public events; and close public institutions. These control measures will help COVID‐19 infected countries to prevent the epidemic effectively. Future research will focus on improving the accuracy of early diagnostic tests, developing the vaccine and identifying effective drugs. Therefore, elucidating the pathogenesis of SARS‐CoV‐2 infection is imperative for achieving such goals.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML