-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2020; 9(1): 6-10
doi:10.5923/j.ijvmb.20200901.02

HIV/Malaria Coinfection among HIV-Infected Individuals in Calabar, Nigeria
John Precious Chidinma1, Cookey Tochi Ifeoma1, Innocent-Adiele Hope Chioma2, Stanley Catherine Nonyelum3, Okonko Iheanyi Omezuruike1
1Virus Research Unit, Department of Microbiology, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria
2Department of Applied Microbiology, Ebonyi State University, Abakaliki, Nigeria
3Department of Pharmaceutical Microbiology & Biotechnology, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria
Correspondence to: Okonko Iheanyi Omezuruike, Virus Research Unit, Department of Microbiology, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Human immunodeficiency virus (HIV) and Malaria are two main global public health threats that dent development in low and middle-income countries. This research assessed the HIV/malaria coinfection among HIV-infected individuals in Calabar, Cross River State, Nigeria. A total of 241 individuals living with HIV from Calabar, Cross Rivers State, Nigeria participated in the study. Their ages ranged from 4-67 years with a mean age of 38.4 years. Plasma samples were analyzed for HIV and Malaria using ELISA. The CD4 count was determined using the Partec CyFlow® Counter. Plasma viral loads (PVL) were obtained using the Abbott Real-Time HIV-1 assay. It was observed that 22.4% of them were in the 36-40 years age range. Most (71.4%) of the HIV-infected individuals were females while 28.6% were males. An overall prevalence of HIV/malaria coinfection in Calabar, Cross River State was 3.0%. A higher HIV/Malaria coinfection rate was observed among age groups <25 years (11.5%) than in other age-groups, and in males (4.3%) than in females (2.3%). Also, higher prevalence of HIV/malaria coinfections was observed in singles (4.7%) than the married (1.9%), and among individuals who had primary education (6.3%) than secondary (6.0%) and tertiary (0.7%). Higher HIV/malaria coinfection was observed among drivers (25.0%) than other occupations. This was followed by students (7.1%), traders (4.8%) and businessmen/women (2.3%) while other occupations recorded zero prevalence. Higher HIV/malaria coinfection was observed among subjects with CD4 cell count <200 cells/μl (7.0%) compared to 350-499 cells/μl (3.9%) and >500 cells/μl (2.1%) while 200-349 cells/μl showed zero prevalence. Higher HIV/malaria coinfection was observed among subjects with PVL >5000 copies/mL (4.1%) compared to 40- 5000 copies/mL (3.0%) and <40 copies/mL (1.3%). This study confirmed the presence of HIV/malaria coinfection in Calabar, Cross River State, Nigeria. This, therefore, emphasizes the need for a well-structured approach to the management of HIV/Malaria co-infection.
Keywords: HIV, Coinfections, Malaria, Nigeria
Cite this paper: John Precious Chidinma, Cookey Tochi Ifeoma, Innocent-Adiele Hope Chioma, Stanley Catherine Nonyelum, Okonko Iheanyi Omezuruike, HIV/Malaria Coinfection among HIV-Infected Individuals in Calabar, Nigeria, International Journal of Virology and Molecular Biology, Vol. 9 No. 1, 2020, pp. 6-10. doi: 10.5923/j.ijvmb.20200901.02.
Article Outline
1. Introduction
- HIV continues to be a major global public health issue, having claimed over 35 million lives so far [1]. In 2018, there were approximately 37.9 million people with HIV/AIDS globally [2] and about 770 000 people passed on as a result of HIV-related causes [1]. Of these, 1.7 million were children (<15 years old) and 36.2 million were adults. Around 21% of these same people do not know that they had the virus [2]. Since the onset of the epidemic, 74.9 million people have been estimated to be infected with HIV and 32 million people have died of illnesses related to AIDS [2].Two of the prevailing infections in sub-Saharan Africa are Malaria and HIV-1 [3]. About 25.7 million Africans are infected with HIV-1 [1-2], while there are 300 million to 500 million cases of malaria each year [4]. Consequently, there will be a significant public health effect if these infections interact together, irrespective of how modest the statistical effect is [3]. With regards to a population basis, an increase in the prevalence of malaria and an increase in parasite density in HIV-infected individuals could lead to an increase in the transmission of malaria affecting both HIV-positive and -negative individuals [5]. It is expected that either infection might impact the clinical course of the other, based on the current understanding of the host immune response to malaria and HIV [3]. Many other types of infections are associated with at least a transient increase in HIV viral load. Hence, it becomes logical to expect malaria to do the same and potentially accelerate the progression of HIV disease [3].Studies carried out in men and nonpregnant women have shown that the underlying epidemiology and intensity of malaria transmission appear to be pertinent for determining the consequences of coinfection. Transmission is intense and continuous in areas of stable malaria despite the occurrence of seasonal variations. In these areas, immunosuppression from HIV infection may amplify rates of malaria infection and clinical malaria disease, however, it does not increase the rates of severe or complicated malaria [3,5-9]. Early in life, immunity develops putting young children and pregnant women at a greater risk of morbidity and mortality from malaria.Although, previous study [10] on the occurrence of HIV and Malaria seropositivity in Port Harcourt, Nigeria indicated no incidence of coinfection. The association between the two infections has important implications. The increase in the risk of clinical malaria in people living with HIV could raise the burden on clinical services in HIV-1 prevalent areas [3]. More data is therefore necessary to document any significant malaria and HIV interactions especially in children. Consequently, the present study sought to determine the prevalence of HIV/Malaria coinfections in HIV-infected individuals residing in Calabar, Nigeria.
2. Materials and Methods
2.1. Study Areas
- The study was conducted at the University of Calabar Teaching Hospital (UCTH) in Cross River State, Nigeria. Cross Rivers State, made up of 18 LGA’s lying between latitude 5° 45’N and longitude 8° 30’E.
2.2. Study Design
- A cross-sectional study in the University of Calabar Teaching Hospital (UCTH) in Calabar, Cross River State, Nigeria was carried out. Approval for the study was gotten from the ethical committee of UCTH. Demographic data and other needed information were collected in a labelled questionnaire form.
2.3. Study Population
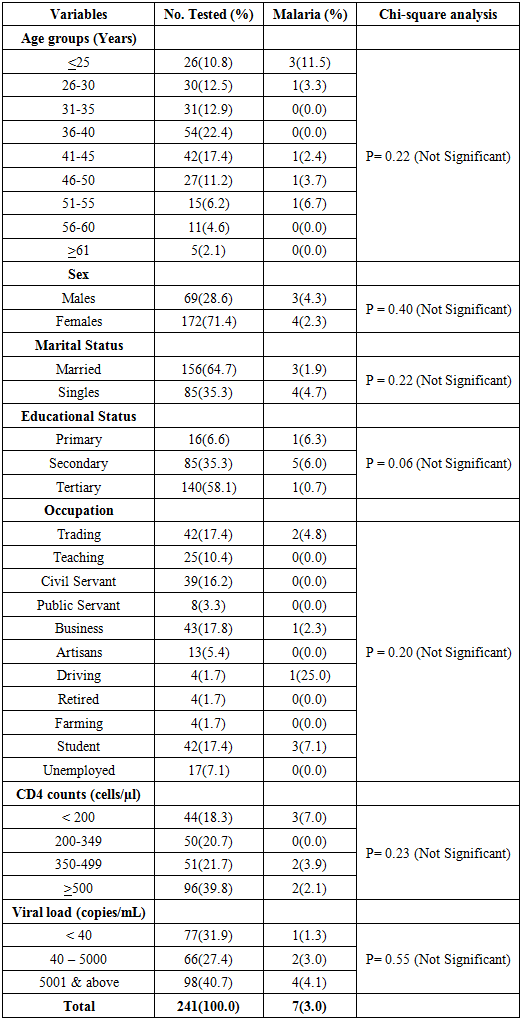
- The study population was HIV- positive subjects attending the University of Calabar Teaching Hospital (UCTH). At most, 241 HIV-positive subjects were selected and enrolled for the study (Table 1). While the entire HIV- positive individuals in Cross River State were the target population to which the findings of the study was extrapolated.
2.4. Serological Analysis of HIV
- All the 417 plasma samples were re-tested using DetermineTM and Stat-pak HIV-1/2 rapid strips to detect HIV-1/2 antibodies (serial algorithm); samples positive to at least, one of the rapid tests were re-tested using 4th generation ELISA (Genscreen Ultra HIV Ag-Ab, Bio-Rad, In-vitro Diagnostics, Raymond Poincare’, France). All seropositive samples were subjected to P24 antigen detection by ELISA following the manufacturer’s specifications.
2.5. Serological Analysis of Malaria
- Plasma samples were analyzed for the presence of Malaria Plasmodium falciparum using the ELISA kit manufactured by DIA.PRO Diagnostic Bioprobes Srl Via G. Carducci n° 27 20099 Sesto San Giovanni (Milano) – Italy, according to manufacturer’s specifications.
2.6. CD4 T Cell Count Enumeration
- EDTA-treated blood samples were used for CD4 T cell count using Partec CyFlow® Counter (Partec GmbH, Munster, Germany) as stipulated by the manufacturer.
2.7. HIV-1 Viral Load Testing (Abbott Real-Time Assay)
- Plasma viral load (PVL) was analyzed using Abbott Real-Time HIV assay US Protocol.
3. Results
3.1. Subjects Characteristics
- A total of 241 HIV-1 infected individuals with ages ranging from 4-67 years (average age = 38.4 years) partook in this study. Most (71.4%) of them were females while 28.6% were males (Table 1). Also, 64.7% were married and 35.3% were singles. Furthermore, 58.1% of the subjects had tertiary education, 35.3% had secondary education and 6.6% had primary education. In terms of occupation, 17.8% were into business, 17.4% were traders and students, followed by civil servants (16.2%), teachers (10.4%), unemployed (7.1%), artisans (5.4%) and public servants (3.3%) while farmers, drivers, and retirees (1.7%) were the least (Table 1).Clinical characteristics of the subjects revealed that CD4 (cells/μl) count ranged from 5 – 2139 cells/μl (average = 473.2 cells/μl) (Table 1). Generally, the plasma viral loads (PVL) ranged from 23 to 3,675,901 copies/mL (average = 158,488 copies/mL) (Table 1).
3.2. Overall Prevalence of HIV/Malaria Coinfection
- Results showed an overall prevalence of HIV/Malaria coinfection to be 3.0%. Table 1 also shows the HIV/malaria coinfection rates amongst HIV-1 infected individuals in Calabar, Nigeria in relation to their sociodemographic and clinical variables.
3.3. Age-specific HIV/Malaria Co-infections
- Higher HIV/Malaria coinfection rate was observed among age groups <25 years (11.5%) than in other age-groups. The age-specific prevalence showed that HIV/Malaria coinfections was highest in ages <25 years (11.5%), followed by ages 51-55 years (6.7%), 46-50 years (3.7%) and 26-30 years (3.3%) while 41-45 years had the least prevalence (2.4%), These differences were not statistically associated (P= 0.22).
3.4. Sex-specific HIV/Malaria Coinfection
- Higher HIV/malaria coinfection was observed among males (4.3%) than in females (2.3%). The study also showed no significant difference (P = 0.40) between sex and HIV/malaria coinfections (Table 1).
3.5. Marital Status-specific HIV/Malaria Coinfection
- Higher HIV/malaria coinfection was observed among individuals who were singles (4.7%) than the married (1.9%). No significant difference (P = 0.22) exist between marital status and HIV/Malaria coinfection (Table 1).
3.6. Educational Status-specific HIV/Malaria Coinfection
- Higher HIV/malaria coinfection was observed among individuals with primary education (6.3%) than other educational status (Secondary 6.0% and Tertiary 0.7%). No significant difference (P = 0.06) exist between educational status and HIV/malaria coinfection (Table 1).
3.7. Occupation-specific HIV/Malaria Coinfection
- Higher HIV/malaria coinfection was observed among drivers (25.0%) than other occupations. This was followed by students (7.1%), traders (4.8%) and businessmen/women (2.3%) while other occupations recorded zero prevalence for HIV/malaria coinfection. No significant difference (P = 0.20) exist between occupation and HIV/malaria coinfections (Table 1).
3.8. CD4-specific HIV/Malaria Coinfections
- Higher HIV/malaria coinfection was observed among subjects with CD4 cell count <200 cells/μl (7.0%) compared to 350-499 cells/μl (3.9%) and >500 cells/μl (2.1%) while 200-349 cells/μl showed zero prevalence (Table 1). These differences were not statistically associated (P= 0.23).
3.9. Viral Loads-specific HIV/Malaria Coinfection
- Higher HIV/malaria coinfection was observed among subjects with PVL >5000 copies/mL (4.1%) compared to 40- 5000 copies/mL (3.0%) and <40 copies/mL (1.3%) (Table 1). These differences were not statistically associated (P= 0.55).
|
4. Discussion
- This study showed that HIV/malaria coinfections was 3.0% which agrees with 3.0% reported in Lagos State, Nigeria by Sanyaolu et al. [11]. It is lower than the 10.3% reported for HIV/Malaria coinfection in Akure, Ondo State, Nigeria [12]. It is also lower than the 4.55% reported in another related study in Akure, Nigeria [13]; the 28.0% reported in Jos [14]; the 4.8% reported in Ethiopia [15]; the 15.5% reported from Ghana [16]; the 21.0% reported in Malawi [17]; the 93.3% reported in HIV-infected Nigerians [18]; the 10.3% reported in Akure, Ondo State, Nigeria [12] and more recently, the 31.0% reported in Northwest Nigeria [19]. However, the 3.0% reported in this study is higher than the 2.24% reported in Bamenda, Cameroon [20]. This difference is most likely because Calabar, Nigeria has low HIV prevalence [21].Higher HIV/Malaria coinfection was observed among age groups <25 years (17.5%) than in other age-groups. Likewise, higher HIV/Malaria coinfection was also observed among age groups <25 years (10.0%) than in other age-groups. This is in correspondence with Jegede et al. [22]. This is different from a study where higher HIV/malaria coinfection was observed in ages of 20-49 years [12]. As observed in eastern sub-Saharan Africa [23,24], age was found to be strongly associated with HIV infection. Gender has been highlighted as a significant risk factor in the frequency of both malaria and HIV coinfections with women being 50.0% more prone to contract malaria than men [25]. The present study contradicts this observation as higher HIV/malaria coinfection was observed among males (5.1%) than females (3.9%). This finding is dissimilar to a study carried out in Akure, Ondo State, Nigeria which reported HIV/malaria co-infection to be higher in females (22.0%) than males (18.9%) [12]. Also contrary to a study in Bamenda Cameroon, higher HIV/malaria coinfection was observed in females than males [20].Higher HIV/Malaria coinfection was observed among individuals who were singles or divorced/widow/widower (7.7%) than in the married individuals (2.3%). This is in agreement with previous studies in Africa [23-24,26-27].Higher HIV/malaria coinfection was observed among individuals who had primary education (7.5%) than other educational status (secondary 4.8%, tertiary 3.5%). This is contrary to many studies which have shown frequency of coinfection to be higher among those with no formal education [28]. No significant difference (P>0.05) exist between occupation and HIV/Malaria coinfections. Higher HIV/Malaria coinfection was observed among HIV-1 individuals with CD4 T cell count <200 cells/μl and 350-499 cells/μl (5.7%), followed by those with CD4 T cell count 200-349 cells/μl (4.9%) and the least prevalence occurred in those with CD4 T cell count >500 cells/μl (2.6%). Tagoe and Boachie [16] reported that malaria co-infection with HIV decreases CD4 T cells and Hb levels in patients. About 39.3% of the infected individuals in this study had high viral loads above 5,000 copies/ml which is indicative of treatment failure, defined by WHO [29] as a persistently detectable viral load exceeding 1000 copies/ml (that is, two consecutive viral load measurements within a three-month interval, with adherence support between measurements) after at least six months of using ARV drugs. Higher HIV/Malaria co-infection was observed among HIV-1 patients with viral loads (VL) above 5000 copies/mL (7.9%) compared to others with 2.0% prevalence. It was revealed that HIV-1 plasma viral loads were high in malaria infected individuals than in those not infected with Malaria by an important study from Malawi, and these levels were found to remain high for up to 10 weeks following treatment [17]. Many factors may be responsible for the variations in HIV and malaria parasite coinfection reported across states and countries. Firstly, geographical difference and other environmental factors play major role in the epidemiological diversity of these diseases as reported in Sub-Saharan Africa [11]. Other factor could be the differences in study design and sample size used, given the fact that the highest prevalence of HIV and malaria co-infection (93.3%) recorded in Port Harcourt, Nigeria [18] was among only 30 HIV infected participants [22]. According to Whiteworth [3], in areas of stable transmission, malaria infection and fever rates become amplified, especially for individuals with low CD4 counts or high viral loads. In contrast, HIV is associated with more severe disease and death in areas of unstable transmission.
5. Conclusions
- This study confirmed the presence of HIV/Malaria coinfection in Calabar, Nigeria. Our findings further highlight the need for a well-structured approach to the management of HIV/malaria coinfection.
ACKNOWLEDGEMENTS
- The authors wish to thank the administrations of University of Calabar Teaching Hospital (UCTH), Calabar, Cross Rivers State, Nigeria, University of Uyo Teaching Hospital (UUTH), Uyo, Akwa-Ibom State, Nigeria for the ethical approvals and all the individuals who participated in this study. Our immense gratitude also goes to Dr. Emeka Michael, University of Uyo Teaching Hospital Uyo, Mr. Inyang Shedrack at the CD4 Unit, University of Uyo Teaching Hospital Uyo, Mrs. Onasochi Shedrack at the Viral Load Unit, University of Uyo Teaching Hospital Uyo, Mr. Usetu Obot, at the PCR Lab, University of Calabar Teaching Hospital Calabar, Nigeria and Miss Immaculate Ugochi Ejike, Virus Research Unit, Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria for their immense contributions during the sample collection and laboratory analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML