-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2018; 7(2): 33-37
doi:10.5923/j.ijvmb.20180702.02

Protease Inhibitor Resistance Mutation Profile in Children on Antiretroviral Therapy for at Least Six Months in Abidjan (Côte d’Ivoire)
Renaud J-J. Dechi1, 2, Thomas A. Toni1, Phillipe J-L. N’din1, 2, Jean F. N’guessan1, Emmanuel Brou1, Ahoua Aguia1, Kouadio Kouakou1, Henri Chenal1, Massara Camara-Cisse2
1Virology Laboratory, Abidjan Integrated Bioclinical Research Center (CIRBA), Abidjan, Côte d’Ivoire
2Biochimistry Laboratory, Faculty of Medical Sciences, University of Félix Houphouët-Boigny (UFHB), Abidjan, Côte d’Ivoire
Correspondence to: Thomas A. Toni, Virology Laboratory, Abidjan Integrated Bioclinical Research Center (CIRBA), Abidjan, Côte d’Ivoire.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Protease inhibitors (PIs) select more mutations than any other class of antiretroviral drugs (ARV). The objective of our study was to determine minor and major mutations in HIV-1 protease that may decrease the efficacy of PIs in children. The determination of mutations and their interpretations were performed using ANRS techniques and algorithms (www.hivfrenchresistance.org). Sequence analysis identified 13% of children resistant to PIs. Frequent minor mutations were M36I and K20I (100% respectively), H69K (88%), L89M and I54V (75% respectively) and G16E (50%). The major mutations were V82A (75%), M46I (63%), L90M (38%) and L76V (13%). Resistance was noted to Indinavir (IDV) and Fosamprenavir/Ritonavir (FPV/r) (75% respectively), Nelfinavir (NFV) and Saquinavir/Ritonavir (SQV/r) (50% respectively), Atazanavir/Ritonavir (ATV/r) (38%) and Lopinavir/Ritonavir (LPV/r) (25%). This study identified mutations associated with PIs resistance in children. The minor mutations frequently encountered whose association with other mutations cause resistance to LPV/r and ATV/r respectively were I54V and G16E. The major mutation responsible for resistance to LPV/r was L76V. The combination of minor and major mutations frequently associated with PIs ineffectiveness was the combination of 4 mutations, I54V, V82A, L90M and M46I for LPV/r and 3 mutations, G16E, L90M and M46I for ATV/r. No resistance to DRV/r was observed, it could be a surrogate molecule.
Keywords: Resistance mutation, Child, HIV-1, Protease inhibitor, Côte d’Ivoire
Cite this paper: Renaud J-J. Dechi, Thomas A. Toni, Phillipe J-L. N’din, Jean F. N’guessan, Emmanuel Brou, Ahoua Aguia, Kouadio Kouakou, Henri Chenal, Massara Camara-Cisse, Protease Inhibitor Resistance Mutation Profile in Children on Antiretroviral Therapy for at Least Six Months in Abidjan (Côte d’Ivoire), International Journal of Virology and Molecular Biology, Vol. 7 No. 2, 2018, pp. 33-37. doi: 10.5923/j.ijvmb.20180702.02.
Article Outline
1. Introduction
- The era of highly active antiretroviral therapy (HAART) began with the introduction of protease inhibitors (PIs) in combination with nucleoside reverse transcriptase inhibitors (NRTIs) [1].HAART has reduced HIV-related morbidity and mortality among children in resource-limited countries [2, 3]. But PIs select more mutations than any other class of antiretroviral drugs (ARV) [4]. The accumulation of these mutations will induce resistance to PIs [1]. Furthermore, the development of broad cross-resistance in this class presents a real challenge in clinical practice [1, 5] especially in children where there are difficulties in the limited number of paediatric dosage forms, adherence, social environment, psychosocial factors and lack of biological monitoring [6].The US Food and Drug Administration (US-FDA) has approved the marketing of 8 PIs [7]. In Côte d'Ivoire, only one is used in children [8]. It is therefore essential to monitor resistance mutations that may affect sensitivity to PIs in a context where therapeutic combination choices remain limited and the number of children on HAART is increasing.With very little up-to-date data on mutations associated with PIs ineffectiveness in children in Côte d'Ivoire, we initiated this study to determine the minor and major mutations in the HIV-1 protease gene involved in the decreased efficacy of PIs.
2. Materials and Methods
2.1. Study Population
- A sample of children from a prospective national cohort at the Abidjan Integrated Bioclinical Research Centre (CIRBA) from 2012 to 2013, including 260 children infected with HIV-1, constituted our study population. The children included in the study had HIV-1 or HIV-1+2 positive serology, were under 18 years of age, had started and been on ARV therapy for at least 6 months at CIRBA, and had a viral load greater than 1000 copies/mL. The study was approved by the National Research and Ethics Committee (CNER).
2.2. Determination of Mutations in the Protease Gene
- The determination of minor and major mutations on the protease gene was performed using ANRS AC11 reference techniques (http://www.hivfrenchresistance.org). To do this, the protease gene was amplified after extraction of viral RNA with the QIAamp Viral RNA Mini Kit (Qiagen, Germany). The Titan One Tube RT-PCR System (Roche Diagnostics, Manheim, Germany) was used for the first PCR and the Expand™ High Fidelity PCR System (Roche Diagnostics, Manheim, Germany) for the second PCR. The amplicons obtained were purified with the QIAquick PCR Purification Kit (Qiagen, Germany). The sequence reaction was performed with the BigDye™ Terminator v3.1 Cycle Sequencing Kit (Applied Biosystem, Courtaboeuf, France). The products of the sequence reaction were precipitated and purified by an ethanol purification process according to the manufacturer's recommendations (Applied Biosystem, Courtaboeuf, France). An electrophoretic migration of the purified products was done with the Genetic Analyser sequencer 3130 (Applied Bio system, Courtaboeuf, France) for sequence determination. The sequences obtained were aligned with the HIV-1 reference (HIV-1 HXB2, accession number GenBank: K03455) using SeqScape 3.0 software (Applied Biosystem, Courtaboeuf, France). The list of mutations defined is that of the International Aids Society (IAS, http://www.iasusa.org). The interpretation was made using the ANRS algorithm (http://www.hivfrenchresistance.org/, September 2016, Version 26).
2.3. Phylogenetic Analyses
- A In order to specify the viral subtypes, the consensus sequences obtained were aligned with the reference sequences available in the GenBank (http://hiv-web.lanl.gov/). The sequences were aligned with Clustal W [9] software implemented in the BioEdit v7 program [10]. The phylogenetic trees were made with Mega 7 software [11].
2.4. Data Processing and Statistical Analysis
- The SPSS Statistics 17.0.1 software was used for statistical analyses.
3. Results
3.1. Study Patient Characteristics
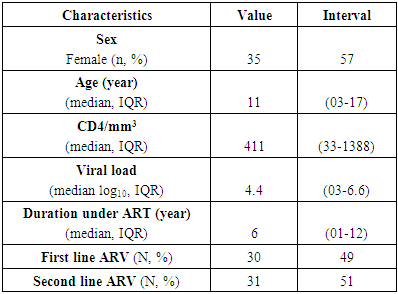
- From a cohort of 260 children, 61 were included in our study, or 23% (n=61/260). Women represented 57% (n = 35/61) of the studied population. The median age was 11 years (03-17 years). The proportion of children on PIs was 51% (n= 31/61). The median viral load was 4.4 log10 copies/mL (03-6.6) with a median CD4 count of 411/mm3 (33-1388). The median duration of treatment was 6 years (01-12) (Table 1).
|
3.2. Distribution of Viral Subtypes
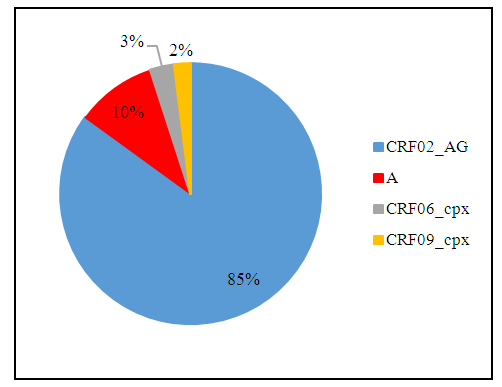
- The synthesis of the different phylogenetic analyses showed that CRF02_AG represented 85% (n= 52/61) of the isolated viral subtypes. Then, subtypes of the viral population, A, CRF06_cpx and CRF09_cpx represented 10% (n = 6/61), 3% (n = 2/61) and 2% (n = 1/61) respectively. Non-B strains of HIV-1 represented 100% (n= 61/61) of the viral subtypes observed (Fig. 1).
3.3. Distribution of Mutations on the Protease Gene
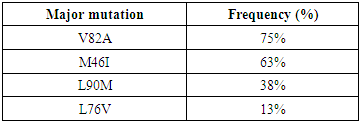
- Analysis of the 61 sequences identified 56 substitutions of which 93% (n=52/56) were minor mutations and 7% (n=04/56) were major mutations. There were 24 mutations associated with resistance. Minor mutations frequently associated with resistance were M36I (substitution of a methionine in position 36 by an isoleucine) and K20I (substitution of a lysine in position 20 by an isoleucine) with a respective frequency of 100% (n= 8/8), H69K (substitution of a histidine in position 69 by a lysine) 88% (n= 7/8), L89M (substitution of a leucine in position 89 by a methionine) and I54V (substitution of an isoleucine in position 54 by a valine) with a respective frequency of 75% (n= 6/8) and G16E (substitution of a glycine in position 16 by a glutamate) 50% (n= 4/8). There were 8 children with PIs resistance, representing a prevalence of 13% (n=8/61). The major resistance mutations and their frequencies are presented in Table 2. The presence of V82A (substitution of a valine in position 82 by an alanine) 75% (n= 6/8), M46I (substitution of a methionine in position 46 by an isoleucine) 63% (n= 5/8), L90M (substitution of a methionine in position 36 by an isoleucine) 38% (n= 3/8) and L76V (substitution of a leucine in position 76 by a valine) 13% (n= 1/8) was noted.
|
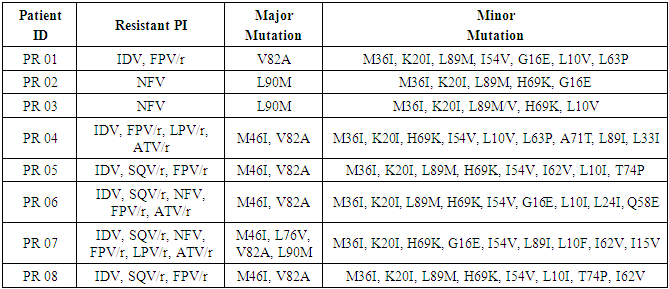
3.4. Genotypic Profiles and Resistant PIs in Children in Failure
- Genotypic profiles and PIs resistant in children are presented in Table 3. All children with resistance had in common the minor mutations M36I and K20I. H64K was present in 7 children (PR 02, 03, 04, 05, 06, 07 and 08), L89M and I54V were found in 6 children (PR 01, 02, 03, 05, 06, 08 and PR 01, 04, 05, 06, 07 08 respectively) and G16E in 4 children (PR 01, 02, 06 and 07). PR 07 had all major mutations (V82A, M46I, L90M and L76V) encountered in our study. PR 04, 05, 06 and 08 had in common the major mutations M46I and V82A. PR 02 and 03 had only one major mutation, the L90M. PR01 also had only one major mutation, V82A.
|
3.5. PIs Resistance Frequency
- Among children with PIs resistance, 2 were in the first line of treatment. Their treatment regimens at the time of failure were DDI+3TC+EFV and AZT+3TC+EFV, respectively. There were 6 children in the second line of treatment who were PIs resistant. Of these, 3 were on a regimen at the time of failure ABC+DDI+LPV/r. Two others had ABC+3TC+LPV/r as their therapeutic regimen. The latter had as therapeutic regimen AZT+DDI+ LPV/r.No resistance to TPV/r and DRV/r was observed. Resistance was noted to IDV and FPV/r with a respective frequency of 75% (n= 6/8), NFV and SQV/r with a respective frequency of 50% (n= 4/8), ATV/r 38% (n= 3/8) and LPV/r 25% (n= 2/8). The frequency of PIs resistance is shown in Figure 2.
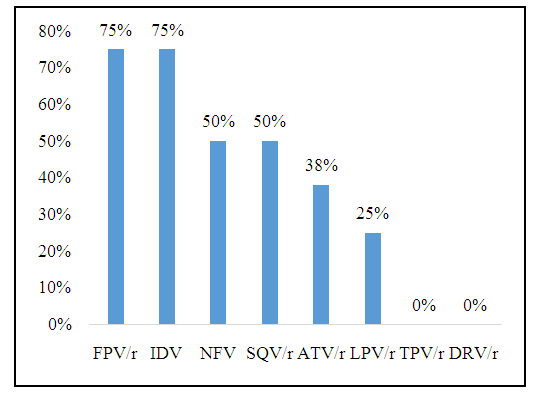 | Figure 2. HIV-1 PIs resistance frequencies in the 8 resistant children included in the CIRBA study in 2012 and 2013 |
4. Discussion
- Phylogenetic analysis of the protease gene in children revealed a heterogeneous distribution of 4 non-B strains of HIV-1 among which CRF02_AG (85%) was predominant.Our study indicated that children infected with non-B strains of HIV-1 had a high frequency of minor mutations. The most frequent were: M36I, K20I, H69K, L89M, I54V and G16E. The major mutations encountered were V82A, M46I, L90M and L76V.Our results are in agreement with those of Anejo-Okopi et al., 2014 [12] and Mossoro-Kpinde et al., 2017 [13] who estimate that the minor mutations M36I, K20I, H69K, L89M and G16E are part of the mutations commonly found in non-B strains of HIV-1 and would correspond to the natural polymorphism in the protease gene of these strains. However, accumulation and association with other « minor » mutations, such as codon 10, may affect the sensitivity of some PIs such as SQV/r and ATV/r.The major mutations in our study have been found in other studies of resistance in children. Thus, Rossouw et al., 2015 [3] and Salou et al., 2016 [6] observed all major mutations in our study, namely V82A, M46I, L90M and L76V. Kuhn et al., 2014 [14] identified 3 of the 4 major mutations, namely M46I, V82A and L76V. Giandhari et al., 2015 [15] observed 2 of the 4 major mutations, namely V82A and M46I. All children with resistance shared the minor M36I and K20I mutation profiles characteristic of non-B HIV-1 subtypes. The resistance profiles also included H69K (88%), L89M (75%), I54V (63%), G16E (50%) and V82A (75%), M46I (63%), L90M (38%), L76V (13%) for minor and major mutations respectively.The interpretation of these mutations according to the ANRS algorithm (September 2016) made it possible to associate them with the ineffectiveness of HIV-1 PIs. Thus, M36I is one of the minor mutations associated with other mutations that cause resistance to IDV, FPV/r and TPV/r. Similarly, the addition of K20I to other mutations results in resistance to SQV/r. In the same register, H69K and L89M cause resistance to TPV/r in HIV-1 subtype B. Also the association of I54V with certain mutations leads to resistance to IDV, FPV/r and LPV/r. G16E in combination with other mutations causes resistance to ATV/r. V82A and M46I are responsible for resistance to IDV. L90M and L76V are responsible for resistance to NFV and LPV/r respectively.Among children with resistance, 2 were in the first line of treatment. Their treatment regimens at the time of failure were DDI+3TC+EFV and AZT+3TC+EFV, respectively. There were 6 children in the second row, with therapeutic regimens consisting of ABC+DDI+LPV/r, ABC+3TC+LPV/r and AZT+DDI+ LPV/r for 3, 2 and 1 children respectively. No resistance to TPV/r and DRV/r was observed. Resistance was noted to IDV and FPV/r with a respective frequency of 75% (n= 6/8), NFV and SQV/r with a respective frequency of 50% (n= 4/8), ATV/r 38% (n= 3/8) and LPV/r 25% (n= 2/8). In Côte d'Ivoire, LPV/r and ATV/r are the 2 PIs commonly used in children. The prevalence of resistance to these drugs remains moderately high. The resistance developed by children in the first line would probably be the result of resistant viruses transmitted. Children in the second line were on an LPV/r regimen at the time of failure. Resistance to ATV/r, IDV, FPV/r, NFV and SQV/r would be cross-resistance.
5. Conclusions
- This study confirmed the prevalence of non-B strains of HIV-1, particularly CRF02_AG. It also identified minor and major mutations in the HIV-1 protease gene associated with PIs resistance in children in Côte d'Ivoire. LPV/r and ATV/r are the 2 PIs commonly used in children infected with HIV-1. Some minor mutations frequently encountered in our study whose association with other mutations led to resistance to LPV/r and ATV/r respectively. The major mutation responsible for resistance to LPV/r was L76V. No single major mutation was responsible for resistance to ATV/r. The combination of minor and major mutations frequently associated with molecule ineffectiveness was the combination of 4 mutations, I54V, V82A, L90M and M46I for LPV/r and 3 mutations, G16E, L90M and M46I for ATV/r. No resistance to TPV/r and DRV/r was observed. TPV/r is not being available in our context; DRV/r because of its genetic barrier remains a molecule of choice in relay treatments with PIs.
ACKNOWLEDGEMENTS
- We thank all the others members of Abidjan Integrated Bioclinical Research Center (CIRBA), PO Box, 2071, Abidjan 18-Côte d'Ivoire for their reception of the office and cooperation.We thank also the Biochemistry Laboratory, Faculty of Medical Sciences, University of Félix Houphouët-Boigny (UFHB), PO Box, 582 Abidjan 22-Côte d’Ivoire.We would like to express our deepest gratitude to Strategic Support Program for Scientific Scientist (PASRES). PO Box 1303 Abidjan 01-Côte d'Ivoire for financing this study.
Abbreviation
- ID: patient identity, PI: protease inhibitor, IDV: Indinavir, FPV/r: Fosamprenavir/ Ritonavir, NFV: Nelfinavir, LPV/r: Lopinavir/Ritonavir, ATV/r: Atazanavir/Ritonavir, SQV/r: Saquinavir/ Ritonavir, V: Valine, A: Alanine, L: Leucine, M: Méthionine, I: Isoleucine, K: Lysine, G: Glycine, P: Proline, H: Histidine, T: Thréonine, Q: Glutamine, TPV/r: tipranavir/ ritonavir, DRV/r: Darunavir/ritonavir.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML