-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2018; 7(1): 11-25
doi:10.5923/j.ijvmb.20180701.02

Prevalence of Adenovirus and Coxsackievirus B4 in Fresh Produce Plants from Egypt
Ahmed R. Sofy 1, Khalid A. El-Dougdoug 2, Adel A. Mousa 1, Ghada S. E. A. Salem 3, Ahmed A. Hmed 1, Amr R. Ghalleb 1, 3
1Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt
2Virology Lab., Agric. Microbiology Department, Faculty of Agriculture, Ain Shams University, Cairo, Egypt
3Food Hygiene Department, Animal Health Research Institute
Correspondence to: Khalid A. El-Dougdoug , Virology Lab., Agric. Microbiology Department, Faculty of Agriculture, Ain Shams University, Cairo, Egypt.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Viruses have been increasingly recognized as important causes of foodborne disease. One category of implicated foods is those that are minimally processed, such as fresh produce. The initial attachment of enteric viruses to green vegetables is a critical step in the chain of contamination events. Therefore, the primary target of this research is study of the prevalence of enteric viruses (Adenovirus and Coxsackievirus B4) to draws attention to the threat of viruses as a risk to public health and accordingly, can provide insight into appropriate intervention methods that can be used to either prevent attachment or remove the attached viral pathogens. A total of 135 leafy green vegetables samples from three governorates (Giza, Kafr El-Sheikh, and Qalyubia) were collected, where 45 samples from each governorate. The vegetable items which chosen for the current study were lettuce, spinach, and green onions, where 15 samples from each item. From our findings, it was found that Adenovirus was present in some examined vegetables samples from the three locations but in different prevalence contamination percentages. Lettuce, spinach and green onion were contaminated by 13.3%, 20% and 13.3% for fields irrigated with water from Elmaryotia canal (Giza governorate), respectively. While, by 60%, 46.6% and 26.6% for fields irrigated with water from the Kitchener drain (Kafr El-Sheikh governorate), respectively. As well as, by 33.3%, 13.3% and 6.6% for El-Gabal El-Asfar farm (Qalyubia governorate), respectively. On the other hand, CoxB4 was not detected in any samples from the region of Elmaryotia canal. In contrast, the three vegetable items of the area of Kitchener drain were contaminated by 40%, 26.6%, and 30%, respectively. In the same trend samples of El-Gabal El-Asfar farm showed contamination percentages were 40%, 26.6%, and 20%, respectively. It is worth mentioning statistically found that, there is a significant difference between locations regarding Adenovirus and Cox B4 present on lettuce plant (p ≤ 0.05). While no statistically significant difference between locations regarding Adenovirus and CoxB4 present on spinach and green onion plants (p > 0.05). However, there was a highly statistically significant difference between locations regarding Adenovirus and CoxB4 at (p ≤ 0.001).
Keywords: Adenovirus, Coxsackievirus B4, Fresh produce, Elmaryotia canal, Kitchener drain, El-Gabal El-Asfar, Egypt
Cite this paper: Ahmed R. Sofy , Khalid A. El-Dougdoug , Adel A. Mousa , Ghada S. E. A. Salem , Ahmed A. Hmed , Amr R. Ghalleb , Prevalence of Adenovirus and Coxsackievirus B4 in Fresh Produce Plants from Egypt, International Journal of Virology and Molecular Biology, Vol. 7 No. 1, 2018, pp. 11-25. doi: 10.5923/j.ijvmb.20180701.02.
Article Outline
1. Introduction
- Viruses are estimated to be the responsible for 59% of the total foodborne illnesses, 27% of the hospitalizations, and 12% of the deaths [1]. Enteric viruses in water and food are a significant cause of mortality in infants and young children in less developed countries [2]. Human enteric virus transmission is known to occur through contaminated food or water (by the fecal-oral route), person to person, aerosols, and contact with contaminated surfaces. Human enteric viruses have a low infectious dose, and a few, as ten virus particles are capable of causing illness [3]. Foods that are associated with transmission include shellfish, fresh produce and ready-to-eat (RTE) foods that do not undergo further processing [4]. The main viruses associated with foodborne diseases are Adenoviruses and other enteroviruses including Coxsackievirus B4 [5].Human adenovirus (HAdV), belonging family Adenoviridae, and are icosahedral, non-enveloped viruses with a double-stranded DNA genome [6]. HAdV includes more than 70 HAdV types, classified into seven species (HAdV A–G) [7, 8]. Human adenoviruses can cause various diseases such as gastrointestinal tract (gastroenteritis), respiratory diseases (acute respiratory infection), eye (conjunctivitis), genitalia (urethritis), and central nervous system (meningoencephalitis) [9]. They have been detected in shellfish, wastewater, and surface waters in many locations [10]. Adenoviruses can spread not only via droplet infection but also via the fecal-oral route. They cause 10% of gastroenteritis in children and are the second most common cause of hospitalization due to diarrhea of children [11].The human enteroviruses are ubiquitous, enterically transmitted viruses that cause a broad spectrum of illnesses among infants and children [12]. Enteroviruses may persist on fresh fruit and vegetables for several days under conditions commonly used for storage in households. Therefore, there will be a risk of infection from consumption of those foods if they are contaminated with viruses. Though enteroviruses are mainly transmitted via the fecal-oral route, the spread of certain species via aerosol is also a cause for concern [13]. Viral particles are shed with feces and symptoms of the diseases caused by them are different from typical gastroenteritis. They enter the host with contaminated food and multiply in the digestive tract. Symptoms of infection are often slight, moderate. However, viruses may spread into other organs and cause diseases that are serious or even fatal such as aseptic meningitis, and occasionally paralysis [14]. Cases of infection giving evidence of association with eating soft fruit, green vegetables and other foods have been recognized [15, 16]. Among enteroviruses, the group B coxsackieviruses, particularly Coxsackievirus B4 (CVB4) [17]. This virus was first isolated in 1948, in New York State during a paralytic poliomyelitis investigation in the town of Coxsackie [18]. CVB4 is a small, nonenveloped, positive-strand RNA enterovirus in the Picornaviridae family, which also includes poliovirus, echovirus, and coxsackievirus A and enterovirus [17]. These viruses cause a wide range of diseases includes gastroenteritis and many other severe illnesses. Therefore, the primary target of this research is drawing attention to the threat of viruses as a risk to public health when they are present in foods (fresh produce) that do not or minimal undergo further processing so, it is important as we seek to develop mitigation and intervention strategies for increment of the efficacy of food safety.
2. Materials and Methods
2.1. Samples Collection
- A total of 135 fresh produce samples were collected from three governorates [45 samples from fields irrigated with water from Elmaryotia (Giza governorate), 45 samples from fields irrigated with water from Kitchener drain (Kafr El-Sheikh governorate), and 45 samples from El-Gabal El-Asfar farm (Qalyubia governorate)]. Three produce items (lettuce, spinach and green onions) were chosen for this study, 15 samples from each item. The samples of each produce were kept in plastic bag and stored at (-20°C) until use for examination and further studies.
2.2. Isolation of Virus
2.2.1. Pre-Analytical Sample Processing
- Fresh plant tissues (2 kg) were sterilized by soaking in 70% ethanol for 5 min then socked in 2% sodium hypochlorite for 1 min, washed in sterilized distilled water for 2 min and dried. To investigate the presence of Human adenovirus (HAdV) and Coxsackievirus B4 (CVB4) in the samples, fresh plant tissues of each sample was cut and mixed in sterile distilled water and macerated using an electric blender. The extraction was filtrated with two layers cheesecloth. The crude sap was clarified by filter paper (Whatman No 4) and stored at -20°C until used for concentration.
2.2.2. Concentration of Virus
- Primary concentration: Viruses were eluted and concentrated from foods surface and crude sap samples via 150 ml of 10% (w/v) beef extract was added to 100 g raw fresh foods and to 100 ml crude sap samples, the mixtures were stirred for 30 min at room temperature and centrifuged at 12000 rpm for 15 min at room temperature [19]. The pellet was discarded, and the supernatant was then concentrated by organic flocculation method.Secondary concentration: All samples were secondary re-concentrated using an organic flocculation method according to Katzenelson et al. [20], Anonymous [21] and Mäde et al. [22], then the elutes were acidified to pH 3.5 using HCl (5N), (Merck-Schuchardt) and centrifuged at 3000 rpm for 15 min. The supernatant was discarded, and the pellet was dissolved in 1ml of Na2HPO4 (El Nasr Pharmaceutical Chemical Co.). All samples were kept at -70°C for further analysis.
2.3. Adenovirus Detection
2.3.1. DNA Extraction & PCR
- DNA extraction was carried out using the phenol-chloroform-isoamyl alcohol method [23], centrifuged at 10,000xg for 5 min, and washed with ethanol 70%. The pellet was resuspended in 40 μl of TE low buffer (pH 8.0) and stored at -20oC before PCR analysis. To exclude any possibility of cross-contamination, all reagents were prepared in a laminar flow cabinet, and all extractions were made in duplicates and processed on different days. The oligonucleotide primers pairs were used according to Allard et al. [24] and Girones et al. [25], where hexAA188518858 5′-GCCGCAGTGGTCTTACATGCACATC-3′ (nucleotides 18858–18883, refers to the Ad2 hexon region), and hexAA191319181 5′-CAGCACGCCGCGGATGTCAAAGT-3′ (nucleotides 19158–19181, refers to the Ad2 hexon region) for conventional-PCR, and nehexAA189318961 5′-GCCACCGAGACGTACTTCAGCCTG-3′ (nucleotides 18961–18937, refers to the Ad2 hexon region), and nehexAA190519108 5′-TTGTACGAGTACGCGGTATCCTCGCGGTC-3′ (nucleotides 19079–19108, refers to the Ad2 hexon region) for nested-PCR. PCR amplification was performed according to Rigotto et al. [26] as follows: 5 μl of DNA and 85 pmoles of each primer (hexAA1885 and hexAA1913) were added to a reaction mix consisting of 20 mM Tris-HCl, pH 8.4, 50 mM KCl, 0.2 mM of dNTPs, 1.5 mM MgCl2, and 5 U of Taq DNA polymerase. Mixtures were incubated for 1 min at 94°C, followed by 40 cycles of 1 min at 94°C, 1 min at 56°C and 45s at 72°C, with a final incubation of 7 min at 72°C, and then, the reaction was held at 4°C. For nested-PCR, 1.0 ml of the first PCR product was added to a 50 ml reaction mix containing 20 mM Tris-HCl, pH 8.4, 50 mM KCl, 0.2 mM of dNTPs, 3.0 mM MgCl2, 85 pmol of each primer (nehexAA1893 and nehexAA1905), and 2.5 U of Taq DNA polymerase. Mixtures were incubated for 1 min at 94°C, followed by 25 cycles of 1 min at 94°C, 1 min at 57°C and 45s at 72°C, with a final incubation of 5 min at 72°C, and then, the reaction was held at 4°C. PCR products were visualized on an ethidium bromide-stained 1.5% agarose gel [26].
2.4. Coxsackievirus B4 Detection
2.4.1. RNA Extraction & RT-PCR
- Viral RNA was extracted directly from concentrated extracts of vegetable samples using a QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. RT-PCR amplified the VP1-2A junction region of Coxsackievirus B4 with primers that flanked the VP1-2A junction region [27]: CoxB4-F3156 5′-CGCATCTACTTCAAACCCAA-3′ (nucleotides 3156–3176, according to Jenkins et al. [28]), and CoxB4-R3468 5′-TTGTGCATTGGCATCTGGC-3′ (nucleotides 3450-3468, according to Jenkins et al. [28]). cDNA synthesis was performed with about 100 ng of RNA using 0.1 μM of the anti-sense CoxB4-R primer and the M-MLV RT enzyme (Promega, Cat #), according to the manufacturer’s instructions. PCR amplification was performed according to Mulders et al. [27] in a final volume of 25 μl as follows: 2.5 μl of cDNA, 2.5 μl of 2.5 mM of dNTPs, 2.5 μl of 10X buffer, 2.5 μl of 25 mM MgCl2, 1 μl of each forward and reverse primers at 10 μM, 0.2 μl Taq DNA polymerase, and water. Mixtures were incubated for 1 min at 94°C, followed by 35 cycles of 1 min at 94°C, 1 min at 42°C and 2 min 30s at 72°C, with a final incubation of 10 min at 72°C, and then, the reaction was held at 4°C. PCR products were visualized on an ethidium bromide-stained 1.5% agarose gel.
2.5. Statistical Analyses
- All statistical calculations were done using SPSS (statistical package for the social science version 25.00) statistical program at the 0.05 level of probability [29]. Qualitative data were done using Chi-square and Pearson correlation. The confidence interval was set to 95% and the margin of error accepted was set to 5%. The P-value was considered non-significant (NS) at the level of >0.05, and significant at the level of <0.05.
3. Results
- A total of 135 leafy green vegetable samples from three governorates (Giza, Kafr El-Sheikh, and Qalyubia) were collected to study the prevalence of enteric viruses (Adenovirus and Coxsackievirus B4) in Egypt. Since 45 samples from fields irrigated with water from Elmaryotia canal (Giza), 45 samples from fields irrigated with water from Kitchener drain (Kafr El-Sheikh), and 45 samples from El-Gabal El-Asfar farm (Qalyubia). The vegetable items which chosen from each studied region were lettuce, spinach, and green onions, where 15 samples from each item.
3.1. Prevalence of Adenovirus
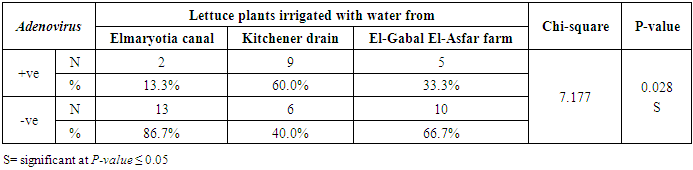
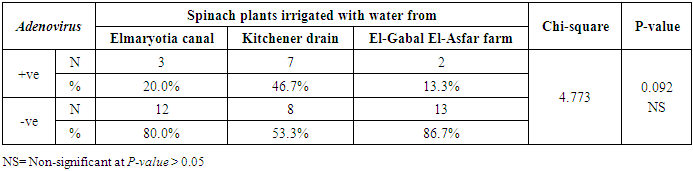
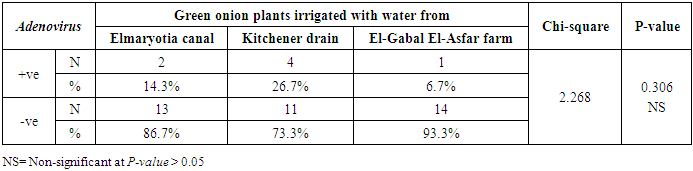
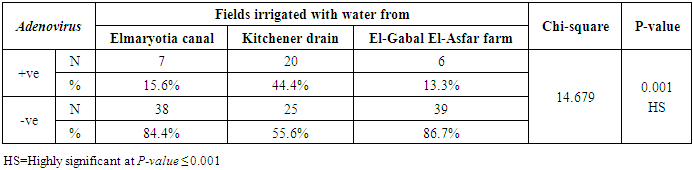
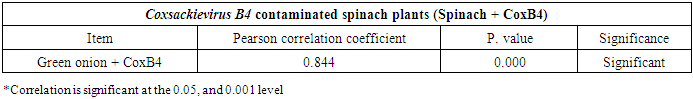
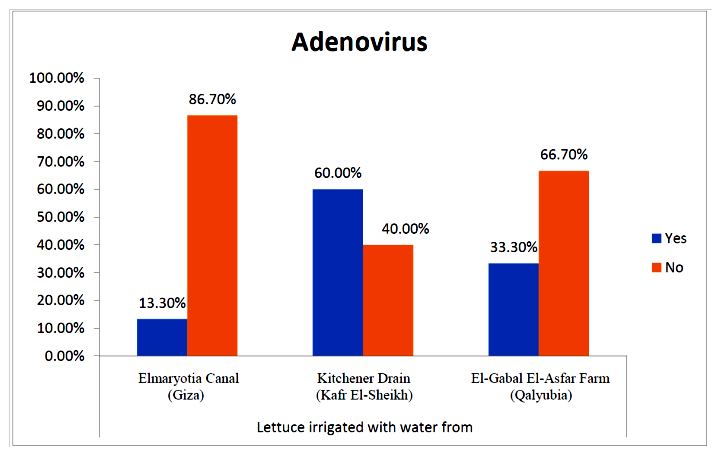
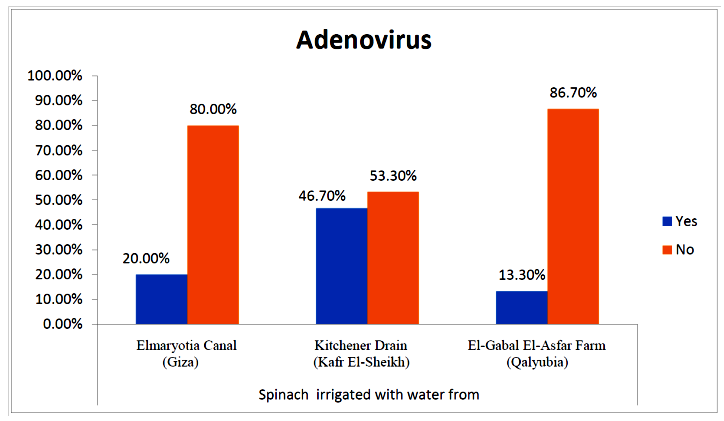
- The results showed that the prevalence contamination percentages of lettuce plants with Adenovirus were 13.3% (2/15), 60% (9/15) and 33.3% (5/15), respectively in the fields irrigated with water from Elmaryotia canal (Giza governorate), Kitchener drain (Kafr El-Sheikh governorate), and El-Gabal El-Asfar farm (Qalyubia governorate) (Table 1 & Fig. 1). On the other hand, the prevalence contamination of spinach plants was 20% (3/15), 46.6% (7/15) and 13.3% (2/15), respectively (Table 2 & Fig. 2). Whereas the prevalence contamination of green onion plants was 13.3% (2/15), 26.6% (4/15) and 6.6% (1/15), respectively (Table 3 & Fig. 3). Since the results revealed the statistically significant difference between locations regarding Adenovirus present on lettuce (p ≤ 0.05), spinach (p > 0.05), and green onion plants (p > 0.05). The highest prevalence of Adenovirus was found in Kafr El-Sheikh governorate by 44.4% (20/45), followed by 15.6% (7/45) in Giza governorate, and 13.3% (6/45) in Qalyubia governorate with high statistically significant difference between locations regarding Adenovirus (p ≤ 0.001) (Table 4 & Fig. 4). On the other hand, the results revealed no statistically significant difference between types of plant regarding irrigated with water from Elmaryotia canal (Giza governorate), Kitchener drain (Kafr El-Sheikh governorate), or El-Gabal El-Asfar farm (Qalyubia governorate) with Adenovirus (p > 0.05).
|
 | Figure (1). Comparison between locations regarding Adenovirus present on lettuce plants |
|
 | Figure (2). Comparison between locations regarding Adenovirus present on spinach plants |
|
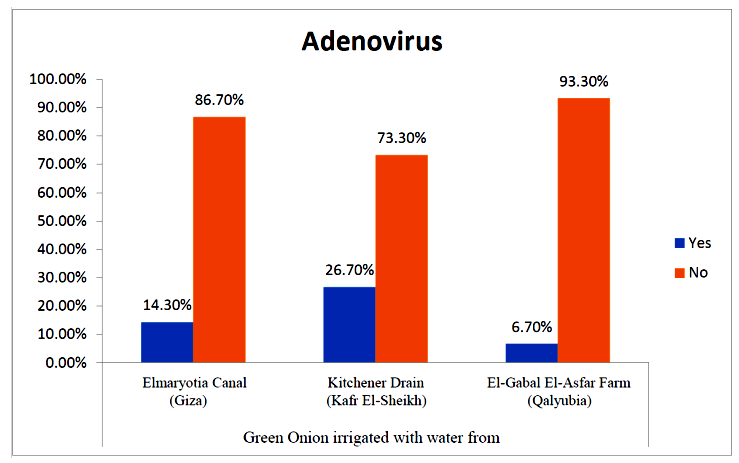 | Figure (3). Comparison between locations regarding Adenovirus present on green onion plants |
|
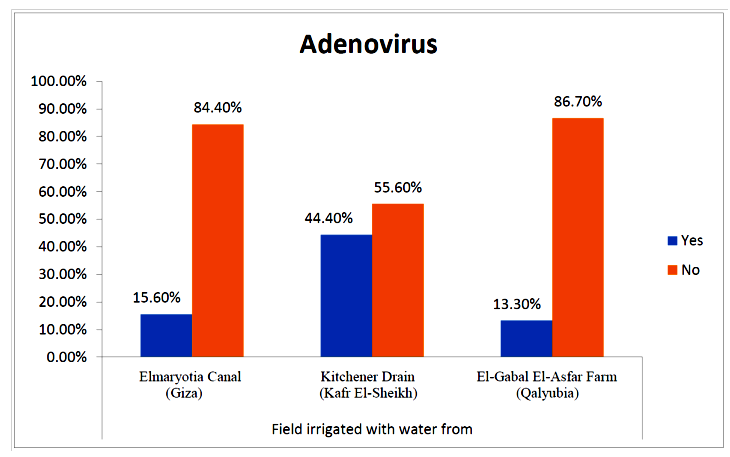 | Figure (4). Comparison between locations regarding Adenovirus |
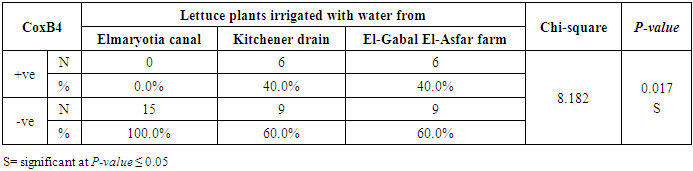
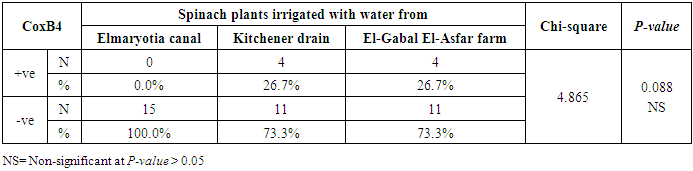
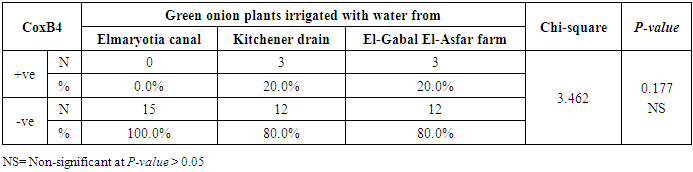
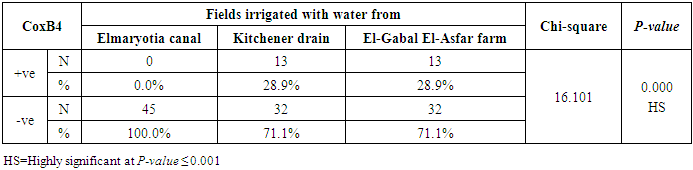
3.2. Prevalence of Coxsackievirus B4
- The results showed that the prevalence contamination percentages of lettuce plants with Coxsackievirus B4 was 40.4% (6/15) in fields irrigated with water from both Kitchener drain (Kafr El-Sheikh governorate), and El-Gabal El-Asfar farm (Qalyubia governorate) (Table 5 & Fig. 5). Also, the prevalence contamination of spinach plants was 26.7% (4/15) in each region (Table 6 & Fig. 6). Whereas the prevalence of contamination of green onion plants was 20% (3/15) in each area (Table 7 & Fig. 7). The results revealed the statistically significant difference between locations regarding CoxB4 present on lettuce plants (p ≤ 0.05), while no statistically significant difference between locations regarding CoxB4 present on spinach and green onion plants. On the other hand, Coxsackievirus B4 was not detected in any samples from the region of Elmaryotia canal (Giza governorate), where the prevalence of Coxsackievirus B4 was found in Kafr El-Sheikh and Qalyubia governorates by 28.9% (13/45) for each governorate with highly statistically significant difference between locations regarding CoxB4 (p ≤ 0.001) (Table 8 & Fig. 8). On the other hand, the results revealed no statistically significant difference between types of plant regarding irrigated with water from Elmaryotia canal (Giza governorate), Kitchener drain (Kafr El-Sheikh governorate), or El-Gabal El-Asfar farm (Qalyubia governorate) with Coxsackievirus B4 (p > 0.05).
|
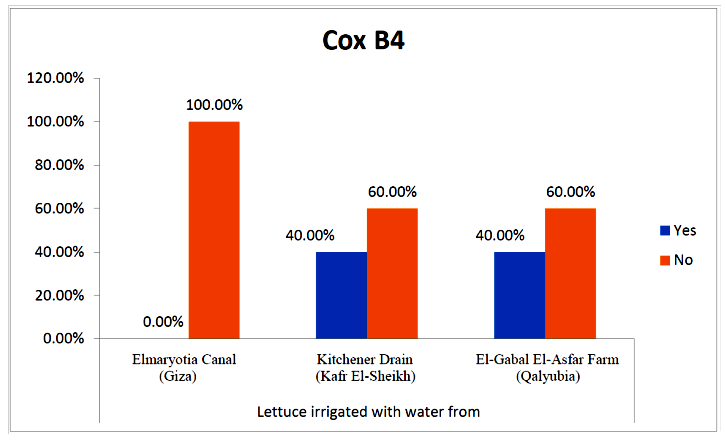 | Figure (5). Comparison between locations regarding CoxB4 present on lettuce plants |
|
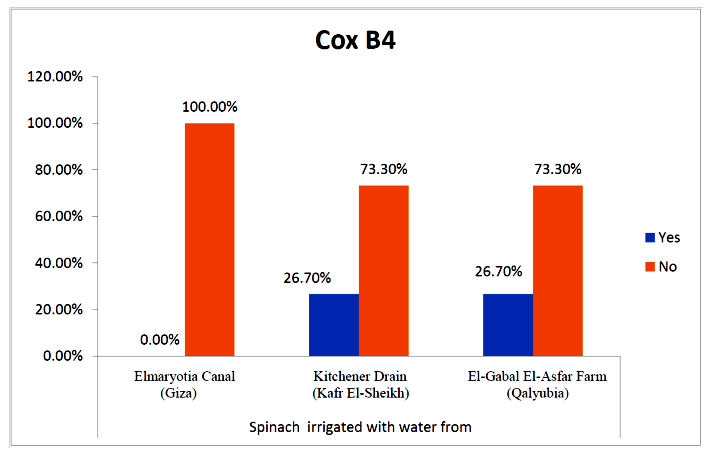 | Figure (6). Comparison between locations regarding CoxB4 present on spinach plants |
|
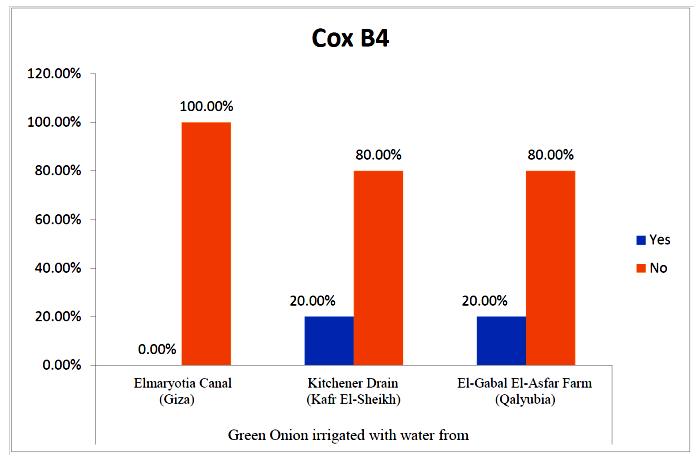 | Figure (7). Comparison between locations regarding CoxB4 present on green onion plants |
|
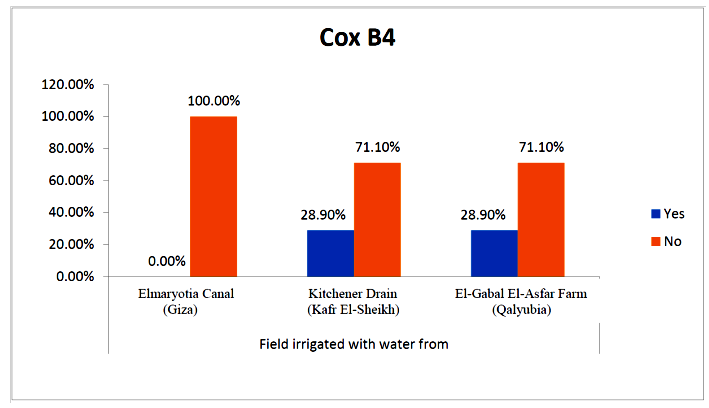 | Figure (8). Comparison between locations regarding CoxB4 |
3.3. Correlation between Adenovirus and Coxsackievirus B4
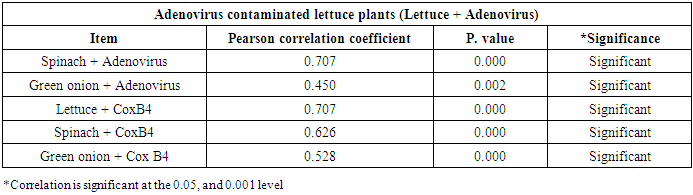
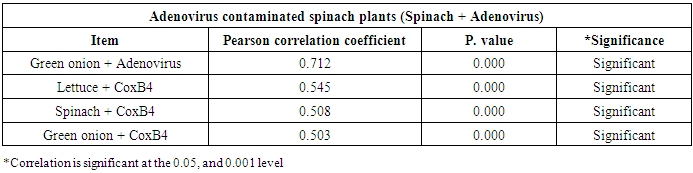
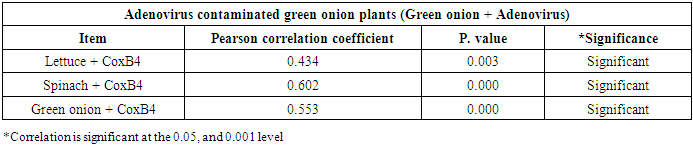
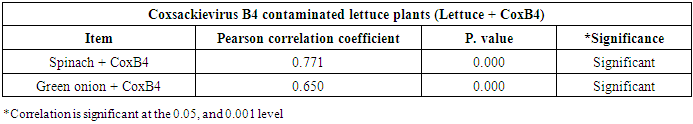
- As shown in tables (9, 10, 11, 12 & 13), there were statistically significant positive correlations as the following: First correlations were between Adenovirus contaminated lettuce plants with Adenovirus contaminated spinach plants (Fig. 9-A), with Adenovirus contaminated green onion plants (Fig. 9-B), with Coxsackievirus B4 contaminated lettuce plants (Fig. 9-C), with Coxsackievirus B4 contaminated spinach plants (Fig. 9-D), and with Coxsackievirus B4 contaminated green onion plants (Fig. 9-E). Second correlations were between Adenovirus contaminated spinach plants with Adenovirus contaminated green onion plants (Fig. 10-A), with Coxsackievirus B4 contaminated lettuce plants (Fig. 10-B), with Coxsackievirus B4 contaminated spinach plants (Fig. 10-C), and with Coxsackievirus B4 contaminated green onion plants (Fig. 10-D). Third correlations were between Adenovirus contaminated green onion plants with Coxsackievirus B4 contaminated lettuce plants (Fig. 11-A), with Coxsackievirus B4 contaminated spinach plants (Fig. 11-B), and with Coxsackievirus B4 contaminated green onion plants (Fig. 11-C). Fourth correlations were between Coxsackievirus B4 contaminated lettuce plants with Coxsackievirus B4 contaminated spinach plants (Fig. 12-A), and with Coxsackievirus B4 contaminated green onion plants (Fig. 12-B). Finally, the last correlation between Coxsackievirus B4 contaminated spinach plants with Coxsackievirus B4 contaminated green onion plants (Fig. 13).
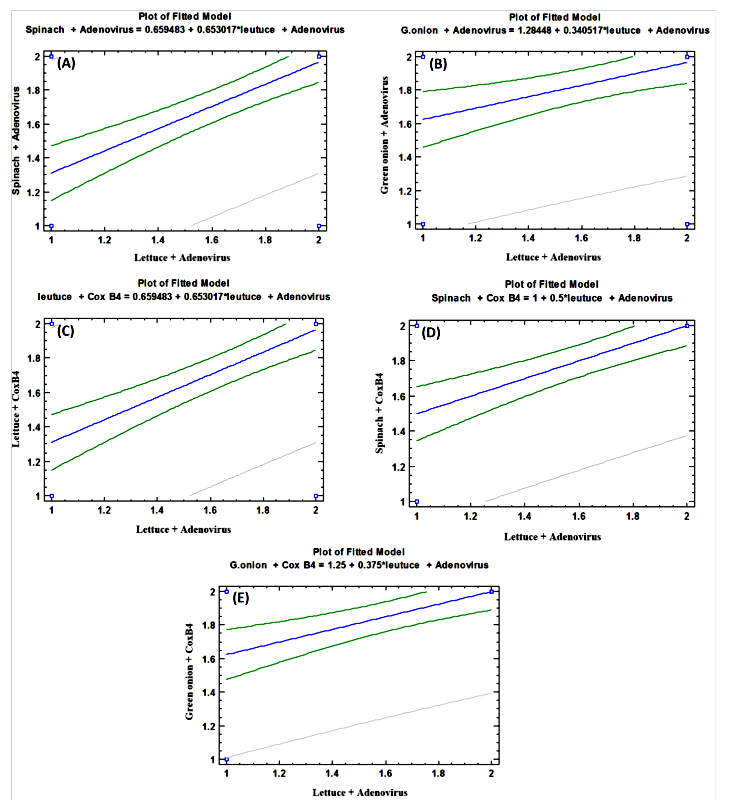 | Figure (9). Linear Pearson Correlation between Adenovirus contaminated lettuce plants with Adenovirus contaminated spinach plants (A), with Adenovirus contaminated green onion plants (B), with CoxB4 contaminated lettuce plants (C), with CoxB4 contaminated spinach plants (D), and with CoxB4 contaminated green onion plants (E) |
|
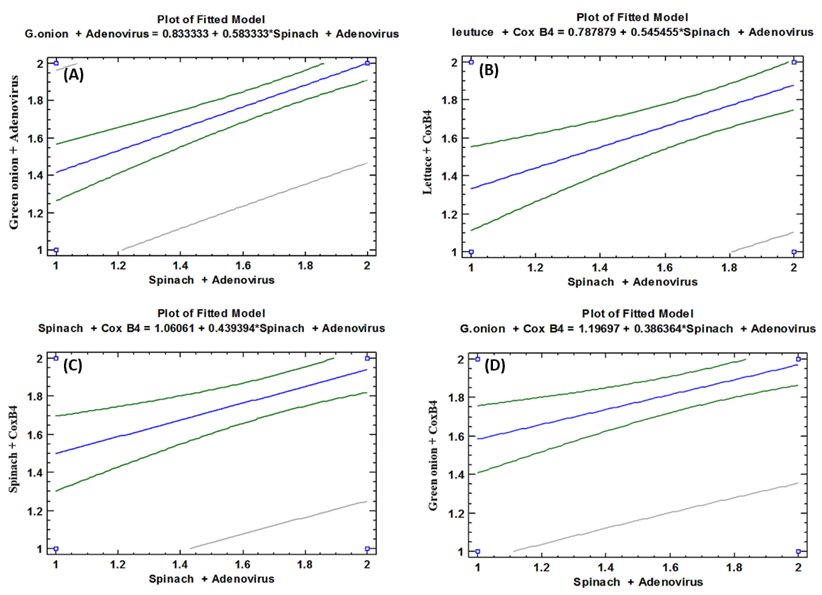 | Figure (10). Linear Pearson Correlation between Adenovirus contaminated spinach plants with Adenovirus contaminated green onion plants (A), with CoxB4 contaminated lettuce plants (B), with CoxB4 contaminated spinach plants (C), and with CoxB4 contaminated green onion plants (D) |
|
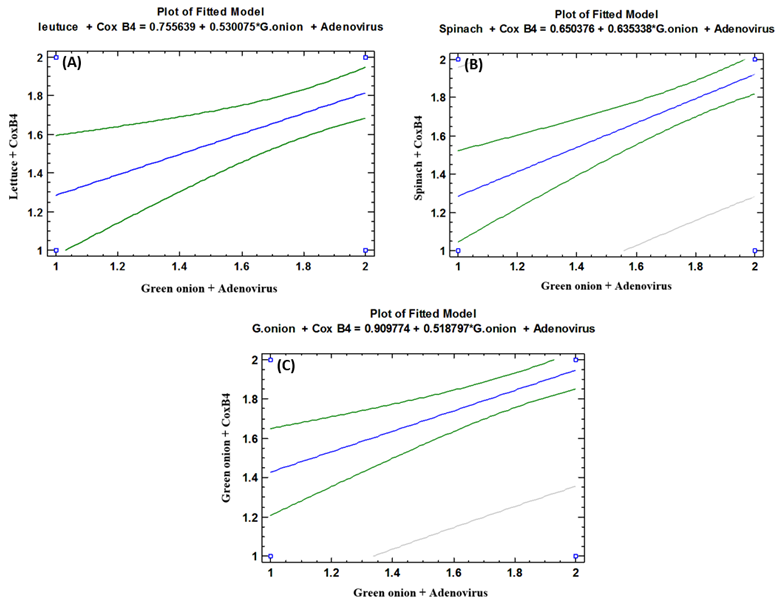 | Figure (11). Linear Pearson Correlation between Adenovirus contaminated green onion plants with CoxB4 contaminated lettuce plants (A), with CoxB4 contaminated spinach plants (B), and with CoxB4 contaminated green onion plants (C) |
|
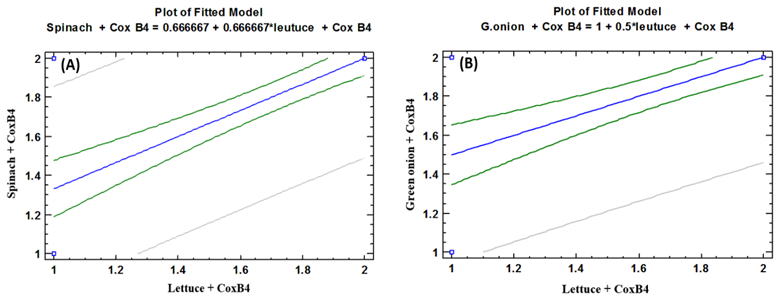 | Figure (12). Linear Pearson Correlation between Coxsackievirus B4 contaminated lettuce plants with CoxB4 contaminated spinach plants (A), and with CoxB4 contaminated green onion plants (B) |
|
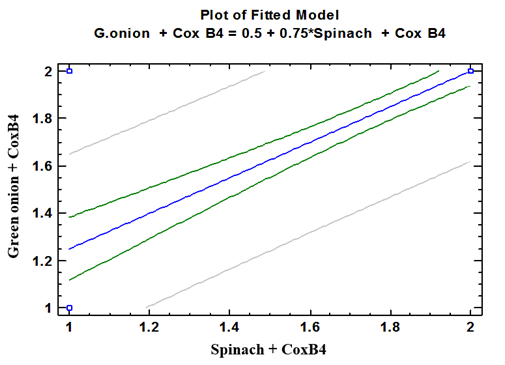 | Figure (13). Linear Pearson Correlation between Coxsackievirus B4 contaminated spinach plants with CoxB4 contaminated green onion plants |
4. Discussion
- Foodborne viruses are transmitted via contaminated food, as well as in combination with person-to-person contact or via contamination of the environment, e.g., water [15]. The contamination of fresh produce with enteric viruses which not subjected to further treatments to be taken must draw attention to the threat of these viruses as a risk to public health and provide insight into appropriate intervention methods that can be used to prevent such contamination with viral pathogens. The obtained results found that Adenovirus was present in some examined vegetables samples from the three locations but in different prevalence contamination percentages. Lettuce, spinach and green onion were contaminated by 13.3%, 20%, and 13.3%, respectively, for fields irrigated with water from Elmaryotia canal (Giza governorate). While by 60%, 46.6% and 26.6%, respectively, for fields irrigated with water from Kitchener drain (Kafr El-Sheikh governorate). As well as, by 33.3%, 13.3% and 6.6%, respectively for El-Gabal El-Asfar farm (Qalyubia governorate). On the other hand, CoxB4 was not detected in any samples from the region of Elmaryotia canal. In contrast, the three vegetable items of the area of Kitchener drain were contaminated by 40%, 26.6%, and 30%, respectively. In the same trend samples of El-Gabal El-Asfar farm showed contamination percentages were 40%, 26.6%, and 20%, respectively. Many foods have been implicated as vehicles in the transmission of enteric viruses. Among the more common ones are shellfish, fruits, vegetables, salads, sandwiches, and bakery items, among others. Indeed, any food that has been handled manually and is not further heated before consumption has the potential for contamination [30].Food contaminated by fecal sources, such as fruits, and vegetables irrigated with water containing sewage or picked by infected workers, and ready-to-eat foods prepared by infected handlers are means of infection with enteric viruses. Enteric viruses cannot multiply in food or water but are generally very environmentally stable outside the host and are acid-resistant such as Human adenovirus and other enteroviruses [15]. Several studies reported that HAdVs are third only to rotaviruses and noroviruses as causative agents of acute gastroenteritis in infants and young children [31]. HAdVs are more frequently associated with acute gastroenteritis, more subtypes explicitly 40 and 41 [32]. Adenovirus transmission occurs by the fecal-oral route, and by ingestion of contaminated food and water [33]. It is known that HAdVs show high stability in the environment [34, 35] and food matrices [34]. As well as, many previous studies have also demonstrated that enteric HAdVs are high disseminated in the aquatic environment and may contaminating vegetables herbs and fruits [36-38]. In addition, a high occurrence of EVs and AdVs was detected in a variety of water environments, including groundwater [39, 40], indicating that raw vegetables can be potentially contaminated with these viruses through irrigation water. Therefore, many studies suggested that, the source of contamination was the discharge of sewage into river water used for irrigating crops. Moreover, Insufficient treatment of sewage can lead to an increase in the numbers of viruses being discharged. Many places worldwide have very limited or no treatment facilities for sewage and consequently semi-treated or raw sewage are pumped straight into the aquatic environment. The role water plays in the epidemiology of HAdV, as well as the potential health risks constituted by these viruses in water environments, is widely recognized [41]. The presence of enteric viruses in sewage and hence in environmental surface waters reflects the infectious status of the population [42] and constitutes a public health risk [43]. In most instances, contamination of fruits and vegetables with enteric viruses is believed to occur before the product reaches food service [44]. Sources of such contamination include the use of contaminated soil, irrigation or washing water, or infected food handlers who harvest the product [45]. Treatment of sewage sludge by drying, pasteurization, anaerobic digestion and composting can reduce but not eliminate viruses, especially the more thermo-resistant ones [46]. Therefore, using recycled sewage effluent and sludge to irrigate or fertilize crops intended for human consumption carries with it the danger of virus contamination [47]. Likewise, soils can also become contaminated by land disposal of sewage sludge and through the use of fecally-impacted irrigation water. Viruses can survive in contaminated soil for long periods of time, the degree to which depends upon factors such as growing season, soil composition, temperature, rainfall, resident microflora, and virus type [48]. El-Senousy et al. [49] reported that Adenovirus may be a suitable candidate viral indicator of human viral contamination in Egyptian water and sewage samples. They recorded the prevalence percentage of Adenoviruses in raw sewage samples collected from Zenin wastewater treatment plant, and Nile water samples collected from El-Giza water treatment plant from July 2009 to June 2011, where was 91.7% (22/24), and 66.7% (16/24), respectively [49].
5. Conclusions and Recommendations
- In conclusion, the obtained results demonstrated that a large number of samples from the three studied areas showed positive results for Human adenovirus and Coxsackievirus B4. Samples from the area of Kitchener drain (Kafr El-Sheikh governorate) showed the highest number of contaminated samples. While, the samples examined from the area of Elmaryotia canal (Giza governorate) showed the lowest number of contaminations, with no detection of CoxB4. So, we can say that vegetables may contain the risk of infection with enteric viruses that may cause serious danger to the public health of humans. Therefore, we recommended that control measures should be targeted at prevention of contamination (e.g., preventive measures at source, sewage treatment, improved food handling). Prevention of environmental sewage and fecal contamination in irrigation of vegetables and crops areas should be a particular focus for risk management activities. Researchers and risk managers need to be on alert to consider the likelihood of foodborne transmission of newly emerging viruses. Studies of the prevalence and levels of virus contamination of foods commonly implicated in outbreaks need to be completed and are essential to enable Quantitative Microbiological Risk Assessment (QMRA) to be conducted.
References
| [1] | Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson M, Roy SL, et al. (2011). Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis, 17: 7-15. |
| [2] | Bernstein DI (2009). Rotavirus overview. Pediatr Infect Dis J, 28: S50–S53. |
| [3] | Center for Disease Control and Prevention (CDC) (2010). Norovirus: Technical Fact Sheet. http://www.cdc.gov/ncidod/dvrd/revb/gastro/norovirus-factsheet.htm. Page last modified on February 23, 2010. Page accessed 1/30/13. |
| [4] | Sair AI, D'Souza DH and Jaykus LA (2002). Human Enteric Viruses as Causes of Foodborne Disease. Comprehensive Reviews in Food Science and Food Safety, 1(2): 73-89. |
| [5] | Hirneisen KA, Black EP, Cascarino JL, Fino VR, Hoover DG and Kniel KE (2010). Viral Inactivation in Foods: A Review of Traditional and Novel Food-Processing Technologies. Comprehensive Reviews in Food Science and Food Safety, 9(1): 3-20. |
| [6] | Benkö M, Harrach B and Russell WC (2000). Family Adenoviridae, p. 227-238. In M. H. V. Van Regenmortel, C. M. Fauquet, and D. H. L. Bishop (ed.), Virus taxonomy. Seventh Report of the International Committee on Taxonomy of Viruses. Academic Press, New York, NY. |
| [7] | Jones MS, Harrach B, Ganac RD, Gozum MMA, Dela CWP, Riedel B, et al., (2007). New Adenovirus species found in a patient presenting with gastroenteritis. J Virol., 81(11): 5978–5984. |
| [8] | Kosulin K, Berkowitsch B and Lion T (2016). Modified pan-Adenovirus real-time PCR assay based on genome analysis of seventy HAdV types. J Clin Virol, 80: 60–61. |
| [9] | Crabtree KD, Gerba CP, Rose JB and Haas CN (1997). Waterborne Adenovirus: A risk assessment. Wat Sci Technol, 35(11–12): 1-6. |
| [10] | Greening GE and Cannon JL (2016). Human and Animal Viruses in Food (Including Taxonomy of Enteric Viruses). In: Goyal S., Cannon J. (eds) Viruses in Foods. Food Microbiology and Food Safety. Springer, Cham. |
| [11] | Vantarakis A and Papapetropoulou M (1999). Detection of enteroviruses, Adenoviruses and hepatitis A viruses in raw sewage and treated effluents by nested-PCR. Water Air Soil Pollut, 114: 85–93. |
| [12] | Acha PN and Szyfres B (2003). Zoonoses and communicable diseases common to man and animals: Volume III: Parasitoses. Pan American Health Organization. http://www.who.int/iris/handle/10665/165519. |
| [13] | Bednar M, Frankova V, Schindler J, Soucek A and Vavra J (1999). Medical Microbiology (in Czech) 1st ed. Prague: Nakladatelstvi Marvil; p. 558. (Reissue). |
| [14] | Formiga-Cruz M, Tofiño-Quesada G, Bofill-Mas S, Lees DN, Henshilwood K, Allard AK, et al. (2002). Distribution of human virus contamination in shellfish from different growing areas in Greece, Spain, Sweden, and the United Kingdom. Applied and Environmental Microbiology, 68(12): 5990–5998. |
| [15] | Koopmans M and Duizer E (2004). Foodborne viruses: an emerging problem. International Journal of Food Microbiology, 90(1): 23–41. |
| [16] | Cook N and Rzezutka A (2006). Hepatitis viruses. In: Motarjemi Y, Adams M. (eds.): Emerging Foodborne Pathogens. Woodhead Publishing Limited, Cambridge. pp. 282-308. |
| [17] | Brilot F, Chehadeh W, Charlet-Renard C, Martens H, Geenen V and Hober D (2002). Persistent infection of human thymic epithelial cells by Coxsackievirus B4. J. Virol., 76(10): 5260–5265. |
| [18] | Committee on Enteroviruses (1962). Classification of human enteroviruses. Virology, 16: 501-504. |
| [19] | Croci L, Dubois E, Cook N, de Medici D, Schultz AC, China B, et al. (2008). Current methods for extraction and concentration of enteric viruses from fresh fruit and vegetables: towards international standards. Food Analytical Methods, 1(2): 73–84. |
| [20] | Katzenelson E, Fattal B and Hostovesky T (1976). Organic flocculation: an efficient second-step concentration method for the detection of viruses in tap water. Applied and Environmental Microbiology, 32(4): 838-839. |
| [21] | Anonymous (2013). Microbiology of food and animal feed: Horizontal method for detection of hepatitis A virus and norovirus in food using real-time RT-PCR: Part 2: Method for qualitative detection Geneva: ISO/TS 15216-2. |
| [22] | Mäde D, Trübner K, Neubert E, Höhne M and Johne R (2013). Detection and typing of norovirus from frozen strawberries involved in a large-scale gastroenteritis outbreak in Germany. Food and Environmental Virology, 5(3): 162–168. |
| [23] | Sambrook J and Russel D (2001). Molecular cloning: a laboratory manual (3rd ed., Vol. 1–3). New York: Cold Spring Harbor Laboratory Press Section. |
| [24] | Allard A, Girones R, Juto P and Wadell G (1990). Polymerase chain reaction for detection of Adenoviruses in stool samples. J. Clin. Microbiol, 28: 2659–2667. |
| [25] | Girones R, Puig M, Allard A, Lucena F, Wadell G and Jofre J (1995). Detection of Adenovirus and enterovirus by PCR amplification in polluted waters. Water Sci. Tech, 31: 351–357. |
| [26] | Rigotto C, Sincero TC, Simões CM and Barardi CR (2005). Detection of Adenoviruses in shellfish by means of conventional-PCR, nested-PCR, and integrated cell culture PCR (ICC/PCR). Water Research, 39(2–3): 297–304. |
| [27] | Mulders MN, Salminen M, Kalkkinen N and Hovi T (2000). Molecular epidemiology of Coxsackievirus B4 and disclosure of the correct VP1/2A(pro) cleavage site: evidence for high genomic diversity and long-term endemicity of distinct genotypes. J Gen Virol, 81(Pt 3): 803-812. |
| [28] | Jenkins O, Booth JD, Minor PD and Almond JW (1987). The complete nucleotide sequence of Coxsackievirus B4 and its comparison to other members of the Picornaviridae. Journal of General Virology, 68: 1835-1848. |
| [29] | Snedecor GW and Chochran WG (1982). Statistical Methods. 7th ed. Iowa State Univ. Press. Iowa, USA. |
| [30] | Richards GP (2001). Enteric virus contamination of foods through industrial practices: a primer on intervention strategies. Journal of Industrial Microbiology and Biotechnology, 27(2): 117-125. |
| [31] | Rezig D, Bahri O, Ben Ayed N, Ben Yahia A, Sadraoui A, Ayed S and Triki H (2006). Identification of Adenoviruses serotypes implicated in haemorrhagic conjunctivitis in Tunisia. Pathol Biol, 54(10): 561-565. |
| [32] | Ghebremedhin B (2014). Human adenovirus: Viral pathogen with increasing importance. Eur J Microbiol Immunol, 4(1): 26-33. |
| [33] | Boone SA and Gerba CP (2007). Significance of fomites in the spread of respiratory and enteric viral disease. Appl. Environ. Microbiol, 73: 1687-1696. |
| [34] | Cheong S, Lee C, Song SW, Choi WC, Lee CH and Kim SJ (2009). Enteric viruses in raw vegetables and groundwater used for irrigation in South Korea. Appl. Environ. Microbiol. 75: 7745-7751. |
| [35] | Hartmann NM, Dartscht M, Szewzyk R and Selinka HC (2013). Monitoring of Adenovirus serotypes in environmental samples by combined PCR and melting point analyses. Virol. J, 10: 190. |
| [36] | Moresco V, Viancelli A, Nascimento MA, Souza DS, Ramos AP, Garcia LA, Simoes CM and Barardi CR (2012). Microbiological and physicochemical analysis of the coastal waters of southern Brazil. Mar Pollut. Bull, 64: 40-48. |
| [37] | Fumian TM, Vieira CB, Leite JP and Miagostovich MP (2013). Assessment of burden of virus agents in an urban sewage treatment plant in Rio de Janeiro, Brazil. J. Water Health, 11: 110-119. |
| [38] | Prado T and Miagostovich MP (2014). Environmental virology and sanitation in Brazil: a narrative review. Cad Saude Publica, 30(7): 1367-1378. |
| [39] | Lee C, Lee SH, Han E, and Kim SJ (2004). Use of cell culture-PCR assay based on combination of A549 and BGMK cell lines and molecular identification as a tool to monitor infectious Adenoviruses and enteroviruses in river water. Appl. Environ. Microbiol, 70: 6695-6705. |
| [40] | Kim SH, Cheon DS, Kim JH, Lee DH, Jheong WH, Heo YJ, et al. (2005). Outbreaks of gastroenteritis that occurred during school excursions in Korea were associated with several waterborne strains of noroviruses. J. Clin. Microbiol. 43: 4836-4839. |
| [41] | Enriquez CE, Hurst CJ and Gerba CP (1995). Survival of the enteric Adenoviruses 40 and 41 in tap, sea, and waste water. Water Research, 29(11): 2548–2553. |
| [42] | Myrmel M, Berg EM, Grinde B and Rimstad E (2006). Enteric viruses in inlet and outlet samples from sewage treatment plants. J Water Health, 4(2): 197-209. |
| [43] | Silva HD, Wosnjuk LAC, Santos SFO, Vilanova-Costa CAST, Pereira FC, Silveira-Lacerda EP, et al. (2009). Molecular detection of Adenoviruses in lakes and rivers of goiˆania, goia’s,Brazil. Food and Environmental Virology, 2(1): 35–40. |
| [44] | Koopmans M, von Bonsdorff CH, Vinjé J, de Medici D and Monroe S (2002). Foodborne viruses. FEMS Microbiol Rev, 26(2): 187-205. |
| [45] | Lopman BA, Brown DW and Koopmans M (2002) Human caliciviruses in Europe. J Clin Virol, 24(3): 137-160. |
| [46] | Metcalf TG, Melnick JL and Estes MK (1995). Environmental virology: from detection of virus in sewage and water by isolation to identification by molecular biology--a trip of over 50 years. Annual Reviews in Microbiology, 49: 461-487. |
| [47] | Ward BK, Chenoweth CM and Irving LG (1982). Recovery of viruses from vegetable surfaces. Applied and Environmental Microbiology, 44: 1389-1394. |
| [48] | Seymour IJ and Appleton H (2001). Foodborne viruses and fresh produce. J Appl Microbiol, 91(5): 759-773. |
| [49] | El-Senousy WM, Barakat AB, Ghanem HE and Kamel MA (2013). Molecular epidemiology of Human adenoviruses and rotaviruses as candidate viral indicators in the Egyptian sewage and water samples. World Applied Sciences Journal, 27: 1235-1247. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML