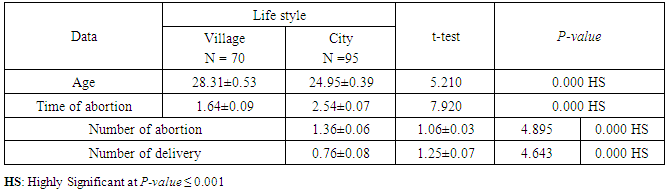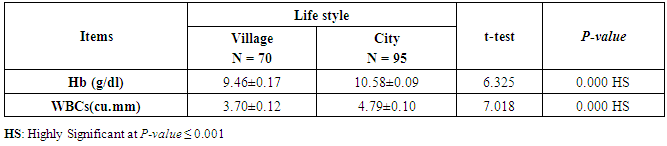-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2017; 6(1): 9-23
doi:10.5923/j.ijvmb.20170601.02

Monitoring and Evaluation of Cytomegalovirus Detection and Risk Factors in Pregnant Women from Egypt
Ahmed R. Sofy 1, Khalid A. El-Dougdoug 2, Adel A. Mousa 1, Mahmoud R. Sofy 1, Ahmed A. Hmed 1, Ahmed A. Abbas 1
1Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt
2Virology Lab., Agric. Microbiology Department, Faculty of Agriculture, Ain Shams University, Cairo, Egypt
Correspondence to: Ahmed R. Sofy , Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2017 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

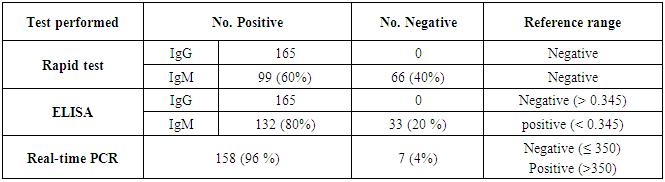
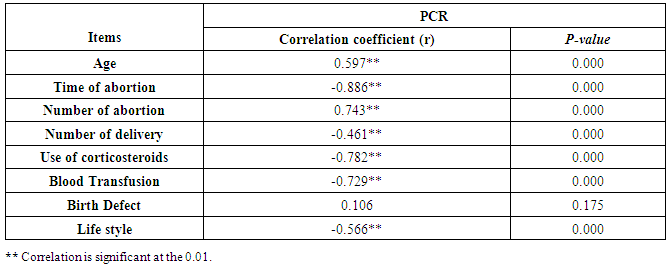
Cytomegalovirus (CMV) is a β herpes virus with double-stranded DNA. Worldwide the current infective rate ranges between 40% and 100%. Fetal loss and abortion are responsible for significant emotional distress for couples desiring children. There are many documents which support the role of Cytomegalovirus (CMV) in spontaneous abortion. A total of one hundred and sixty-five (165) serum samples of pregnant women with anti-CMV IgG positive and with a definite diagnosis of previous abortion were determined. The detection of CMV was done using three methods, rapid chromatographic immunoassay, Enzyme-linked immunosorbent assays (ELISA), and real-time polymerase chain reaction (rt-PCR). The relation between age, time of the abortion, number of abortion, number of delivery, life style, blood transfusion, use of corticosteroids, gynecologic and medical history of abortion, residence and socioeconomic status, against CMV as risk factors of cases were studied. It was found that the best method for detection of CMV was rt-PCR (158/165) positive (96%), then ELISA was (132/165) positive (80%), and the last method rapid test was (99/165) positive (60%), All these methods useful in detection for treatment. Significant positive correlation between PCR, patients ages, number of abortion. While, the negative correlation between PCR and time of the abortion, number of delivery, use of corticosteroids, blood transfusion and life style were found.
Keywords: CMV, ELISA, rt-PCR, Virus, Risk factors, Abortion, Pregnant women, Egypt
Cite this paper: Ahmed R. Sofy , Khalid A. El-Dougdoug , Adel A. Mousa , Mahmoud R. Sofy , Ahmed A. Hmed , Ahmed A. Abbas , Monitoring and Evaluation of Cytomegalovirus Detection and Risk Factors in Pregnant Women from Egypt, International Journal of Virology and Molecular Biology, Vol. 6 No. 1, 2017, pp. 9-23. doi: 10.5923/j.ijvmb.20170601.02.
1. Introduction
- Cytomegalovirus (CMV) was isolated for the first time in 1956 [1]. Weller gave the virus its name from the cytopathic effect produced in cell culture, where the virus causing these cytomegalic cells with intranuclear inclusions was isolated, and hence named Cytomegalovirus [2]. It is a member of the Herpesviridae family, where a β herpes virus with double-stranded DNA [3]. Worldwide the current infective rate ranges between 40% and 100% [4]. One of the previous studies shows that an IgG avidity assay could be used in combination with an IgM ELISA for monitoring pregnant women for primary CMV Infection [5]. While Enzyme-linked immunosorbent assay (ELISA) is one of the best methods for detecting CMV in pregnant women with right diagnoses and treatment [6]. CMV is one of the major causes of congenital infections. Its clinical manifestations range from asymptomatic forms (90% of cases) to severe fetal damage and, in rare cases, death due to abortion. Furthermore, 10%–15% of the children who are asymptomatic at birth may develop late squeal, especially hearing defects, after a period of months or even years [7]. The risk of fetal damage is higher if the primary infection occurs during the first trimester of pregnancy [8, 9]. The prevalence of congenital infection ranges from 0.2% to 2.5% in different populations [10, 11]. Furthermore, the prevalence of congenital infection varies with the prevalence of the infection in the population [12]. As well as increasing with age, may also depend on sexual activity and occupation, particularly occupations involving close contacts with children in a community setting. In the case of parents, contact with the urine or saliva of their children is a major source of infection [13, 14]. The risk of acquiring an infection during pregnancy is 0.7–1.38 % [15]. It is important to evaluate the risk to the pregnant women of being infected and/or symptomatically affected by CMV to provide appropriate counseling and guidance to infection control. So, the aim of this study was to assess for the major causes of CMV among pregnant women at higher risk of getting CMV.
2. Subjects and Methods
- One hundred and sixty-five patients' pregnant women (PW) with repeated abortion happened more than once before. Five ml whole blood obtained under aseptic conditions from each subject by a vein puncture using a disposable syringe. The blood samples divided into two tubes, where the first tube contained EDTA for viral DNA isolation. While the second tube for the serum which was obtained by putting the blood samples in a clean dry plain plastic tube and was allowed to clot at 37ºC for 30 minutes before centrifugation. The tubes centrifuged at 4000 rpm for 5 minutes, and the supernatant (serum) was taken for serology and other tests. A structured interview using a standard maternal questionnaire administered by trained interviewers with the women at their first visit. Questions were asked about the following: age, time of apportion, number of apportion, number of delivery, life style, blood transfusion, use of corticosteroids, gynecologic and medical history of abortion, residence and socioeconomic status. All cases were diagnosed as CMV IgG positive using Rapid Chromatographic Immunoassay Test (RCIT) and Enzyme-linked immune sorbent assays (ELISA) for CMV antigen in the collected serum blood samples according to van der et al. [16] and Nielsen et al. [17], respectively. The ELISA technique performed using kits intended for estimating the concentration of specific CMV-IgM and CMV-IgG markers. The kits were purchased from Sigma Diagnostics (USA), the techniques were performed according to the manufacturer’s instructions. On the other hand, Helicobacter pylori (H. Pylori) assay was done by ELISA using the kits supplied by Diagnostic Products Corporation, USA.Also, CMV was detected by a quantitative real-time PCR. DNA was extracted from samples (first tube) using a Qiagen kit (Qiagen, Valencia, CA, USA) and quantified using the standard laboratory protocol recommended by the manufacturer’s instructions. Qualitative real-time PCR reactions were carried out by using a light Cycler H Instrument (Roche Diagnostics, Meylan, France) with the QuantiTect Probe PCR Kit (Qiagen). Taqman probe (6FAM-CGCGAGACCGTGGAACTGCG-TAMRA), forward primer (5′GCAGCCACGGGATCGTACT-3′) and reverse primer (5′GGCTTTTACCTCACACGAGCATT-3′) were used for amplification according to Coisel et al. [18]. The reaction was carried out in 20 mL, in a final volume containing 10 mL of QuantiTect master mix, 0.2 mM of probe, 0.2 mM of each primer, and 4 mL of DNA. The PCR was initiated by an enzyme-activation incubation at 95 °C for 15 min to activate DNA Polymerase, followed by 40 cycles of denaturation at 95 °C for 10 s and an annealing-extension step at 60 °C for 1 min. Serial dilutions, ranging from 102 to 105 copies/ml of synthesized sequences that correspond to the targeted viral genes, were used as positive controls. These dilutions were also used to determine the viral load in positive samples. A CMV negative specimen was used as a negative control sample was considered positive by real-time PCR if it crossed the threshold.Statistical Data Analysis: For assessment of risk factors for CMV infection (exposure), characteristics of case patients and control subjects examined using a two-sample Student t-test. Cross-tabulation and chi-square or Fisher exact tests were used to examine the relationship between variables using a 95% confidence interval as a measure of association. The sample size was calculated according to Raosoft, and all statistical calculations were done using SPSS (statistical package for the social science version 20.00) statistical program at 0.05 level of probability [19].
3. Results
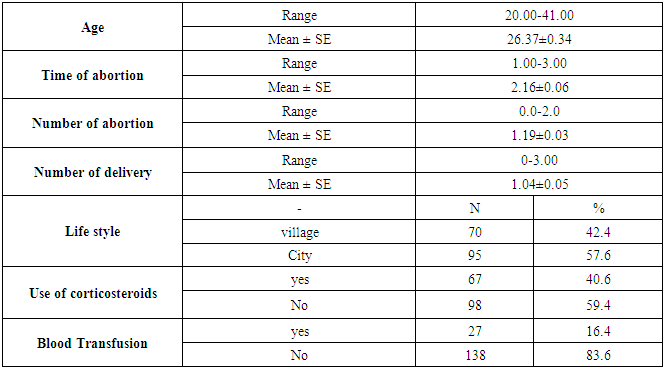
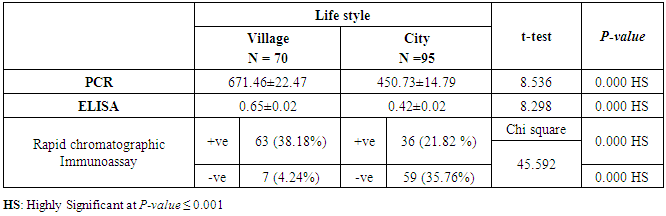
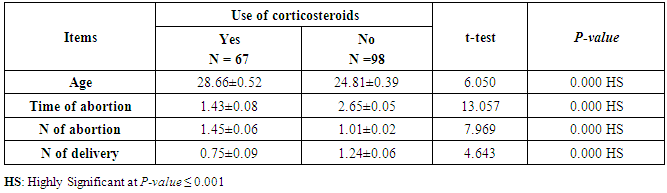
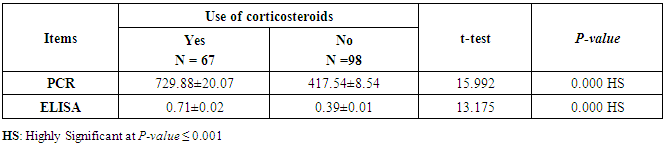
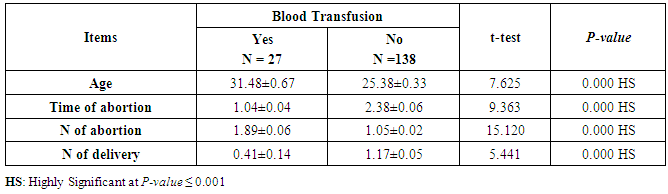
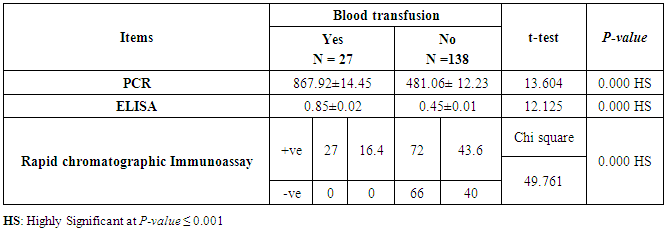
- This case-control study included 165 female patients. Their ages ranged from 20 to 41 years (mean of 26.37±0.34 SE). Their time of abortion, number of abortion and number of delivery ranged from 1 to 3, 0-2 and 0-3, respectively (mean of 2.16±0.06, 1.19±0.03 and 1.042±0.05, respectively). In addition, 70 patient’s village of life style representing 42.4% of female patients and 95 female city of life style representing 47.6% of females patients, 67 females were use of corticosteroids representing 40.6% and 98 females don’t use of corticosteroids representing 59.4% and 27 females exposed to blood transfusion representing 16.4% and 138 females were not exposed to blood transfusion representing 83.6% as shown in table (1).
|
|
|
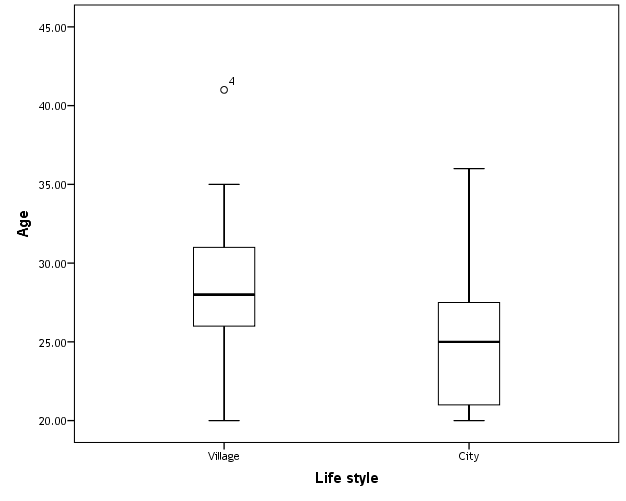 | Figure (1). Box plot of groups as regards age and their statistical difference |
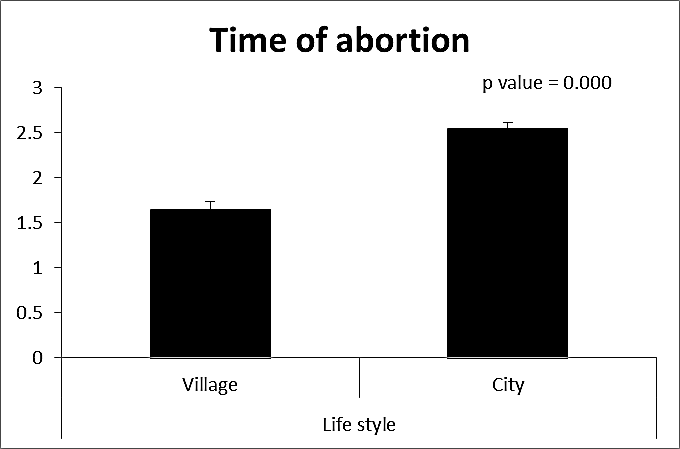 | Figure (2). Comparison between both groups as a time of abortion and their statistical significance |
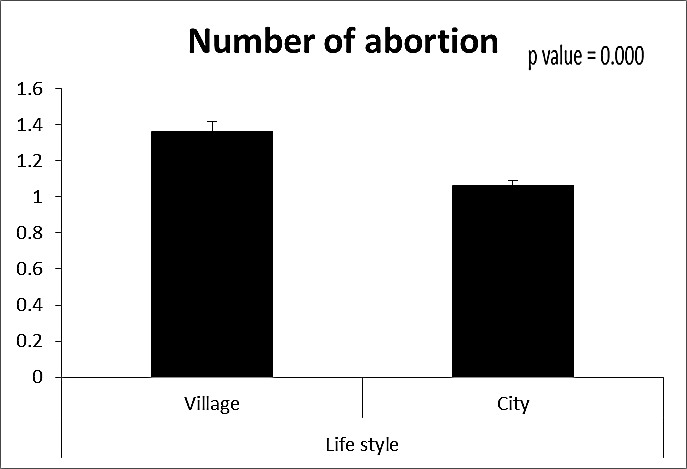 | Figure (3). Comparison between both groups as a number of abortion and their statistical significance |
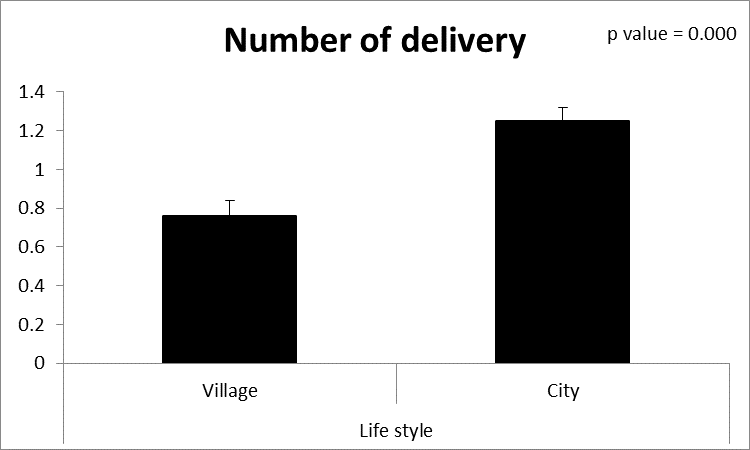 | Figure (4). Comparison between both groups as a number of delivery and their statistical significance |
|
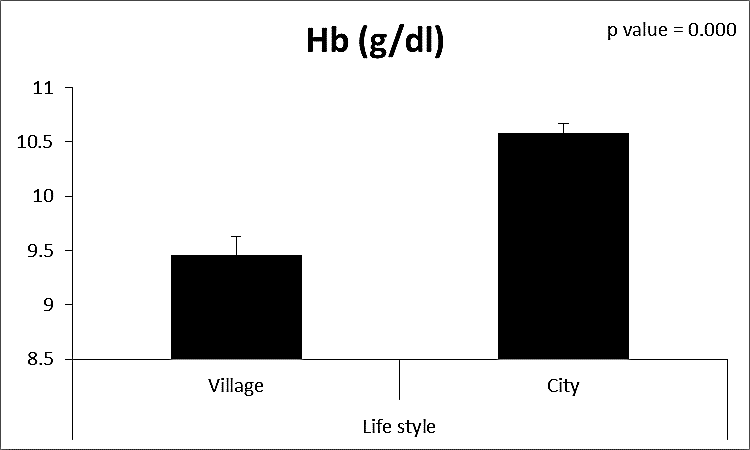 | Figure (5). Comparison between both groups as Hb (g/dl) and their statistical significance |
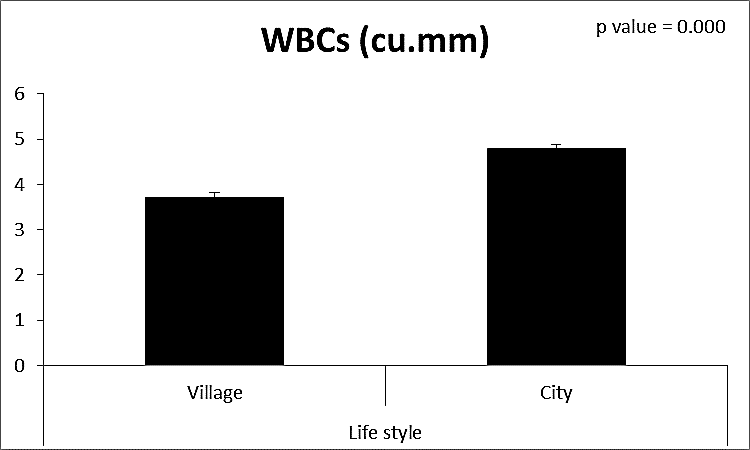 | Figure (6). Comparison between both groups as WBCs and their statistical significance |
|
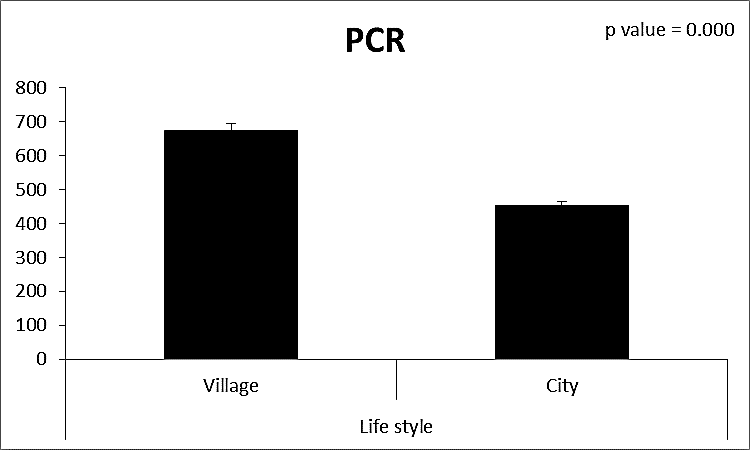 | Figure (7). Comparison between both groups as PCR and their statistical significance |
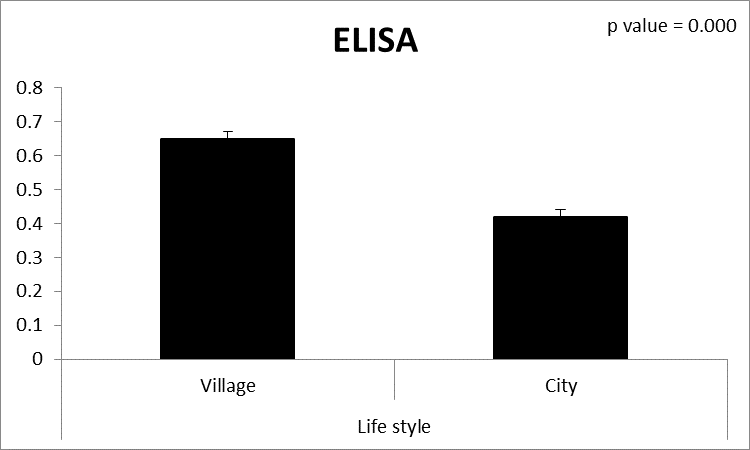 | Figure (8). Comparison between both groups as ELISA and their statistical significance |
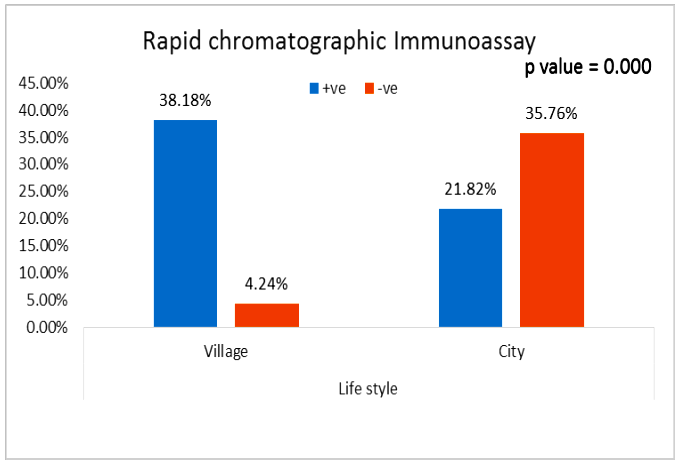 | Figure (9). Comparison between both groups as rapid chromatographic Immunoassay |
|
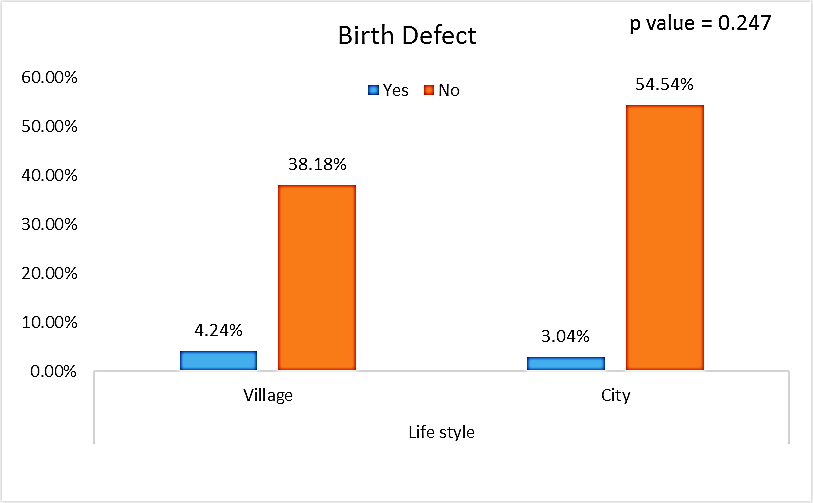 | Figure (10). Comparison between both groups as a birth defect and their statistical significance |
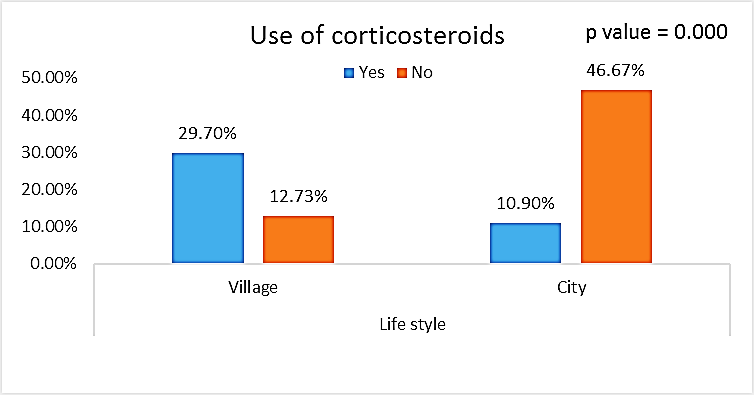 | Figure (11). Comparison between both groups as Use of corticosteroids and their statistical significance |
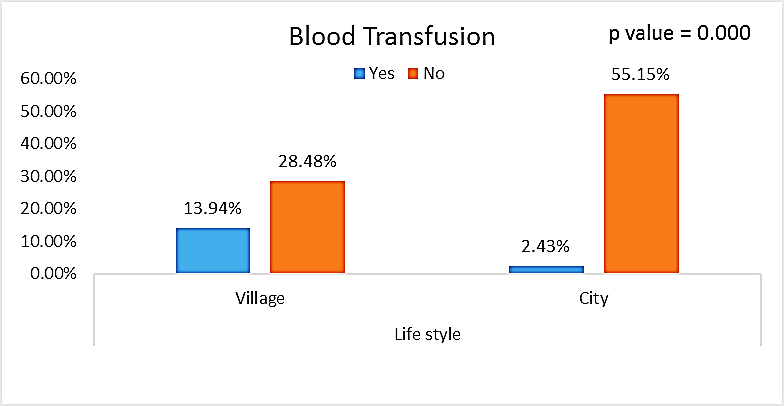 | Figure (12). Comparison between both groups as Blood Transfusion and their statistical significance |
|
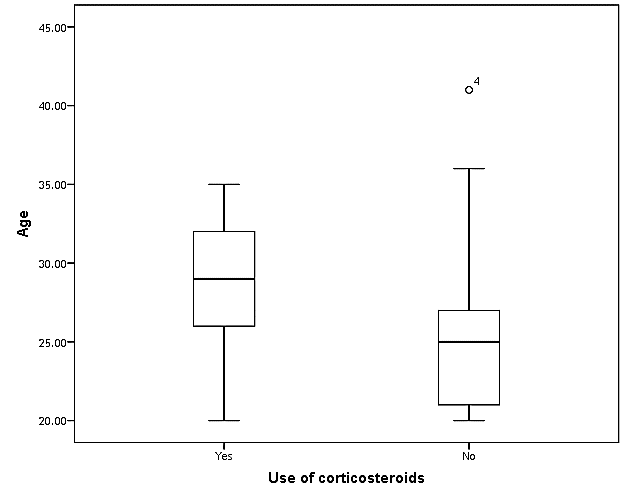 | Figure (13). Box plot of groups as regards age and their statistical difference |
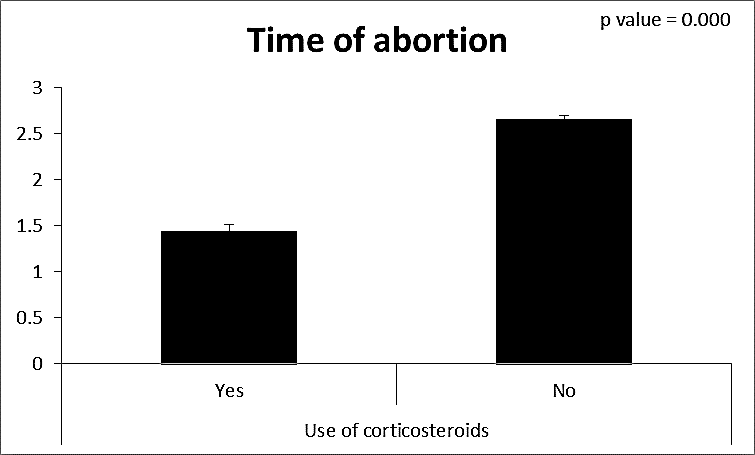 | Figure (14). Comparison between both groups as a time of abortion and their statistical significance |
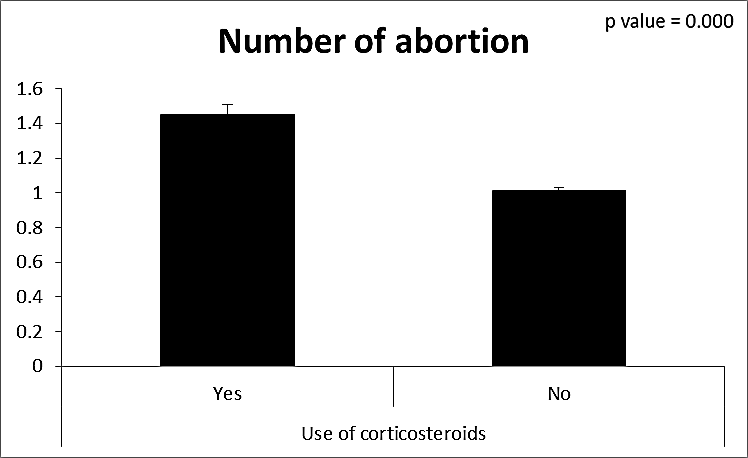 | Figure (15). Comparison between both groups as a number of abortion and their statistical significance |
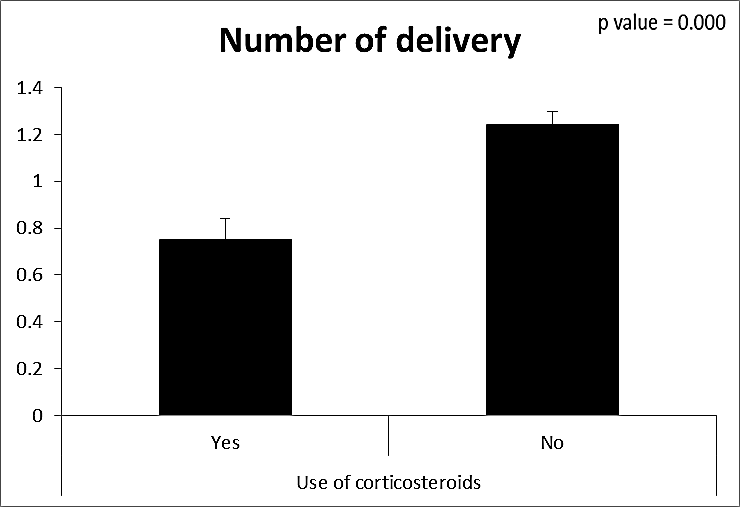 | Figure (16). Comparison between both groups as a number of delivery and their statistical significance |
|
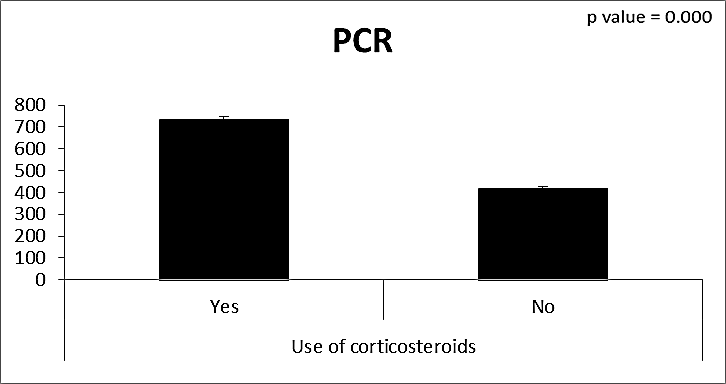 | Figure (17). Comparison between both groups as PCR and their statistical significance |
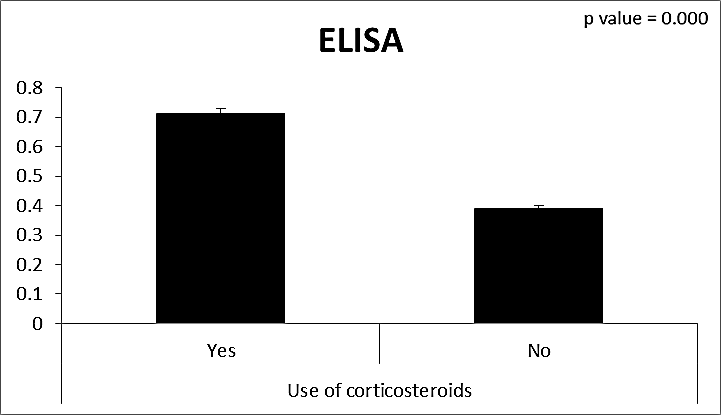 | Figure (18). Comparison between both groups as ELISA and their statistical significance |
|
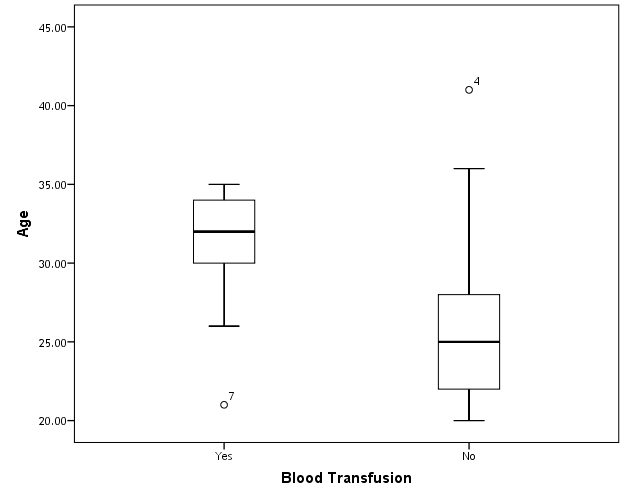 | Figure (19). Box plot of groups as regards age and their statistical difference |
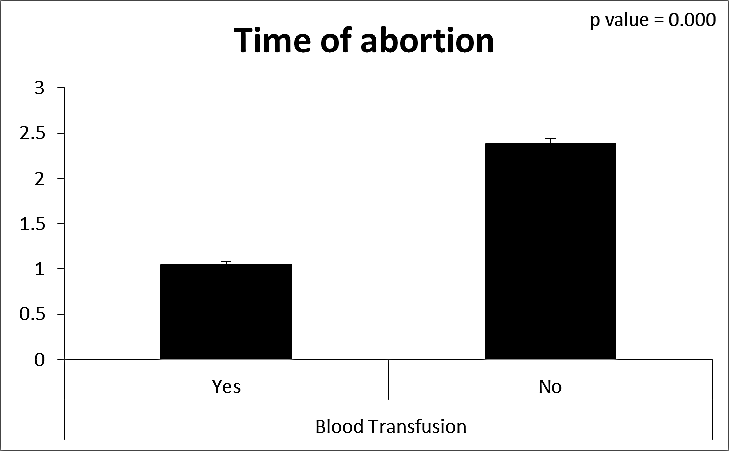 | Figure (20). Comparison between both groups as a time of abortion and their statistical significance |
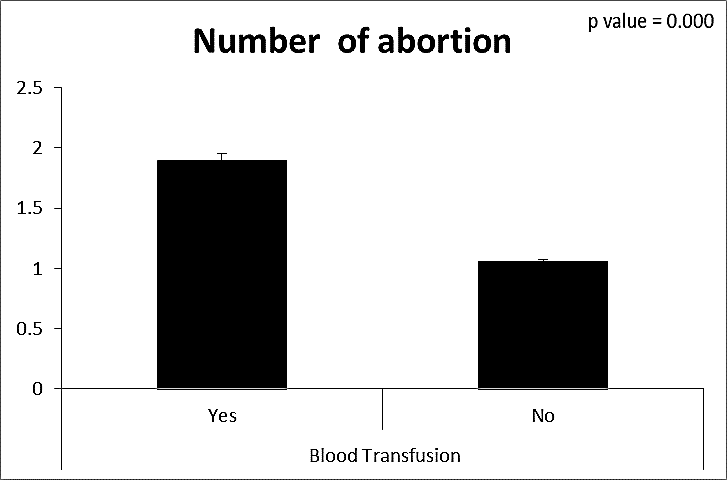 | Figure (21). Comparison between both groups as a number of abortion and their statistical significance |
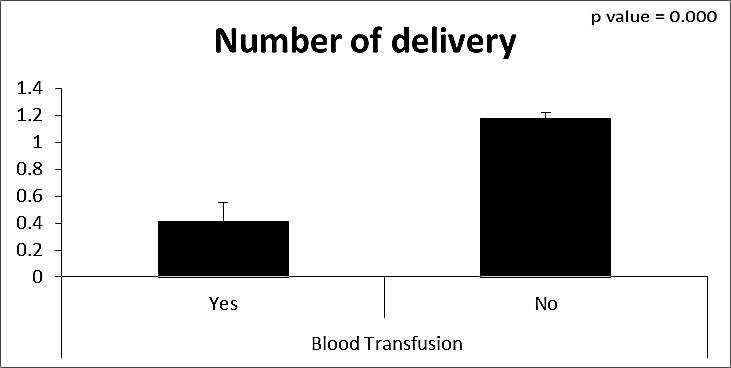 | Figure (22). Comparison between both groups as a number of delivery and their statistical significance |
|
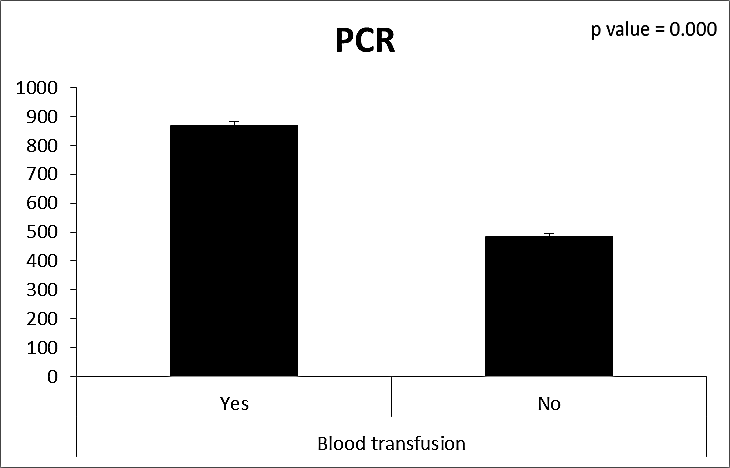 | Figure (23). Comparison between both groups as PCR and their statistical significance |
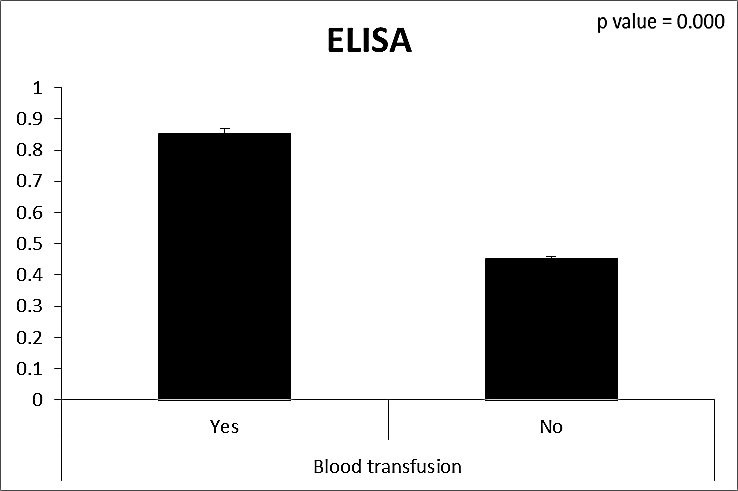 | Figure (24). Comparison between both groups as ELISA and their statistical significance |
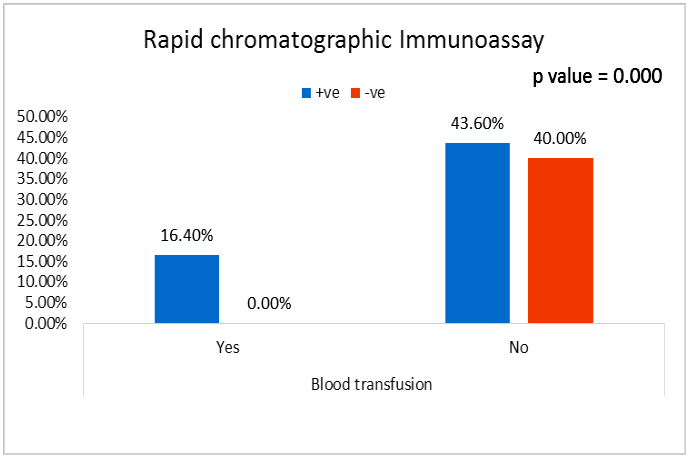 | Figure (25). Comparison between both groups as rapid chromatographic Immunoassay and their statistical significance |
|
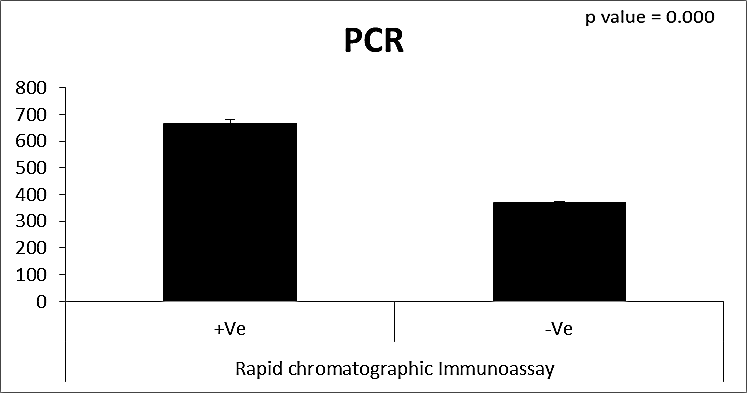 | Figure (26). Comparison between both groups as PCR and their statistical significance |
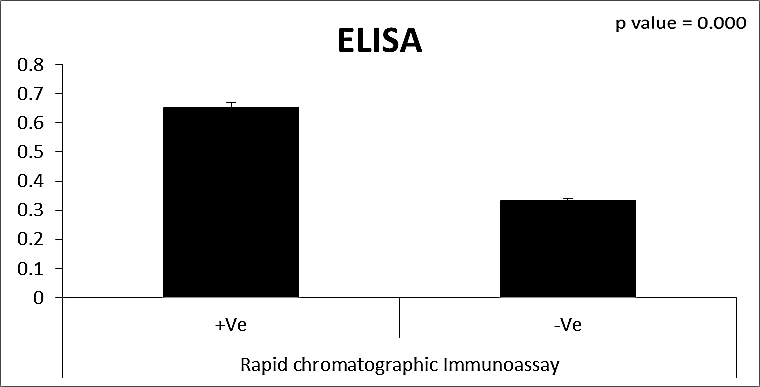 | Figure (27). Comparison between both groups as ELISA and their statistical significance |
|
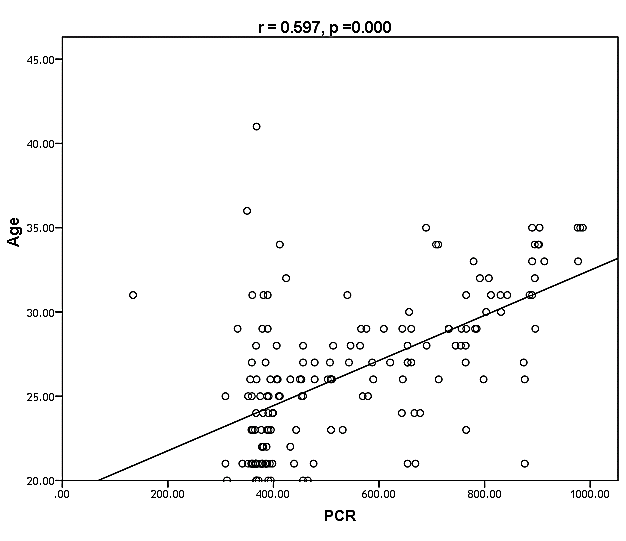 | Figure (28). Linear correlation between PCR, age and their statistical significance |
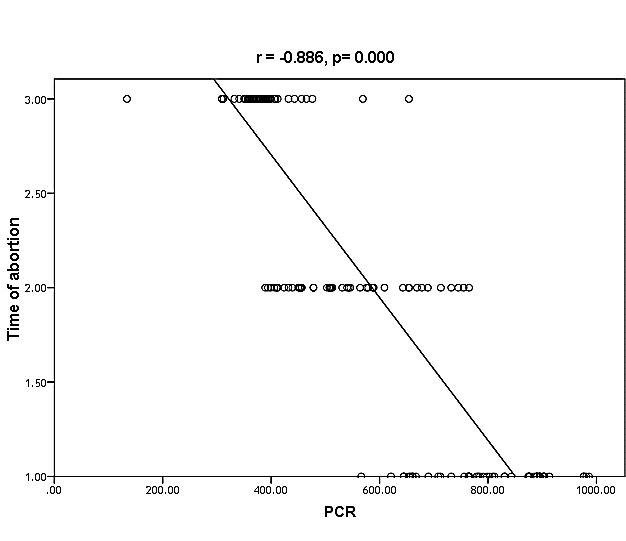 | Figure (29). Linear correlation between PCR, time of abortion and their statistical significance |
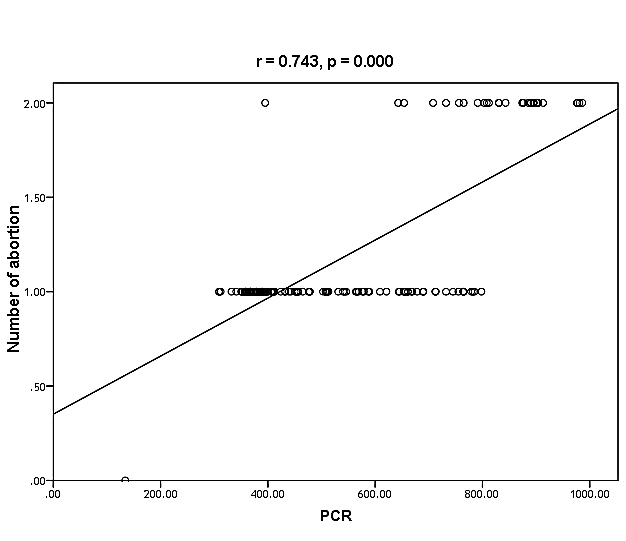 | Figure (30). Linear correlation between PCR, number of abortion and their statistical significance |
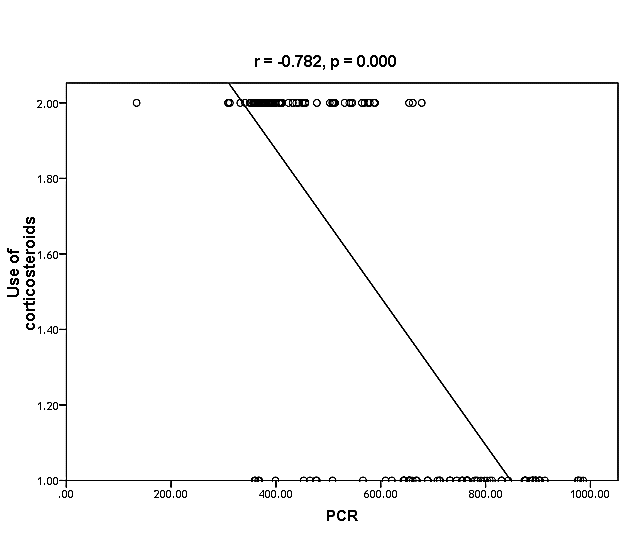 | Figure (31). Linear correlation between PCR, use of corticosteroids and their statistical significance |
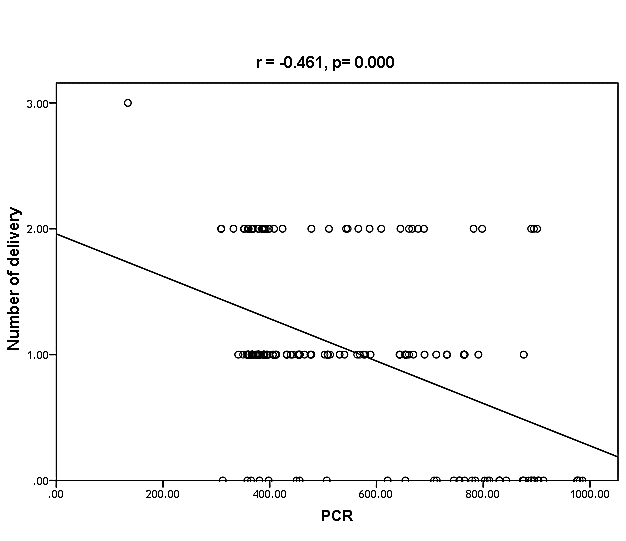 | Figure (32). Linear correlation between PCR, number of delivery and their statistical significance |
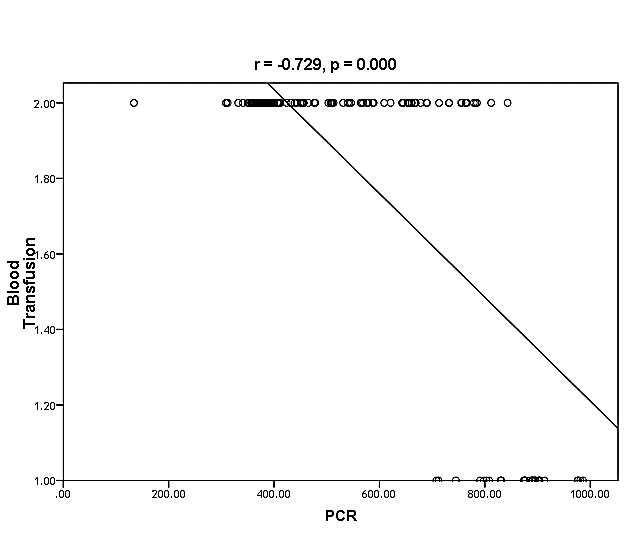 | Figure (33). Linear correlation between PCR, blood transfusion and their statistical significance |
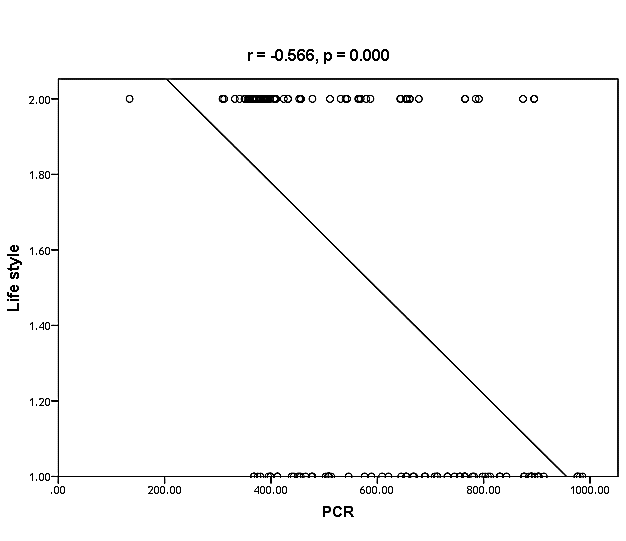 | Figure (34). Linear correlation between PCR, life style, and their statistical significance |
|
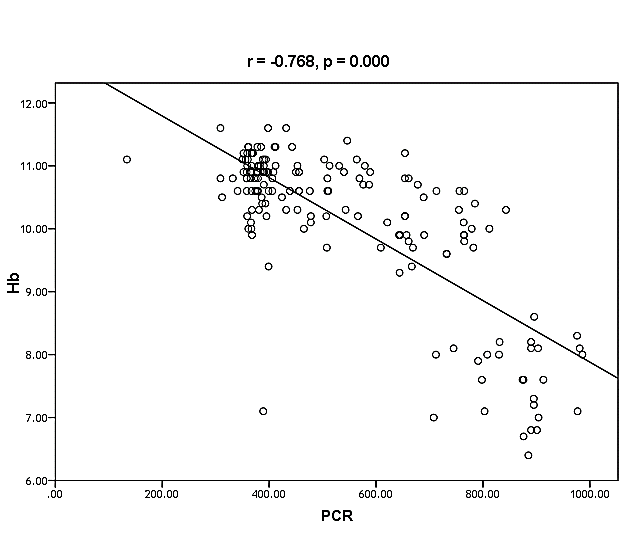 | Figure (35). Linear correlation between PCR, Hb and their statistical significance |
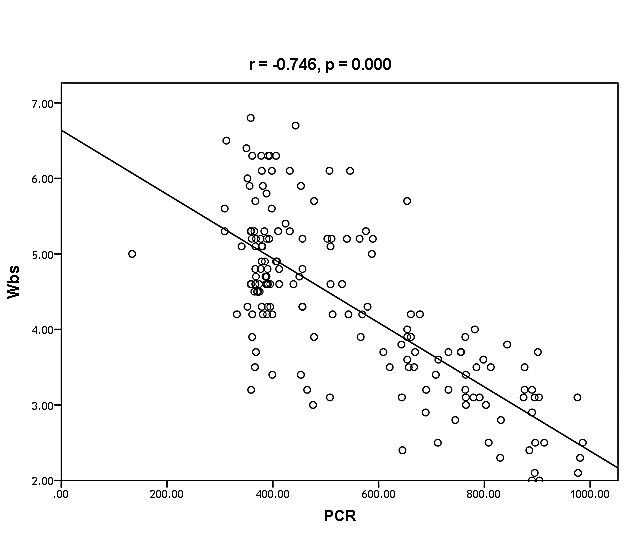 | Figure (36). Linear correlation between PCR, WBCs and their statistical significance |
|
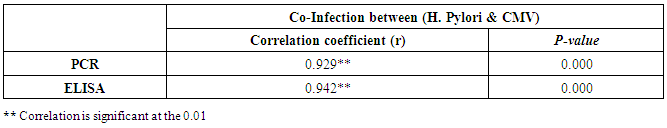
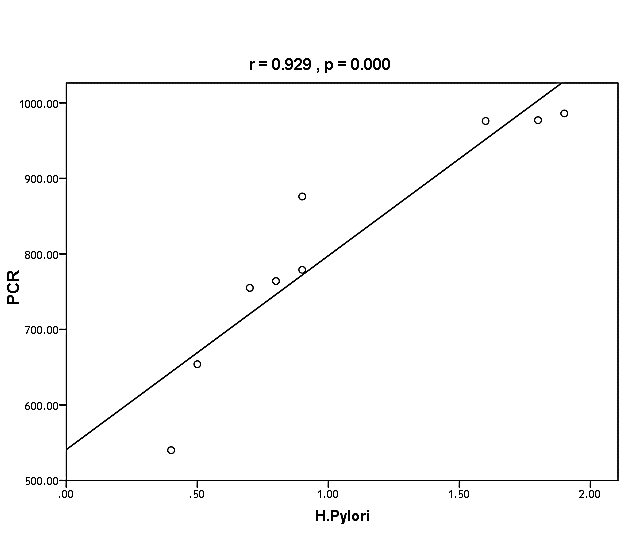 | Figure (37). Linear correlation between H. pylori, PCR, and their statistical significance |
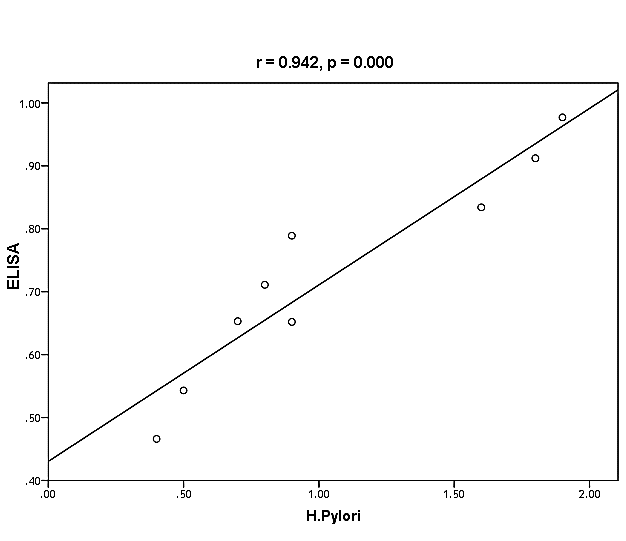 | Figure (38). Linear correlation between H. pylori, ELISA, and their statistical significance |
4. Discussion
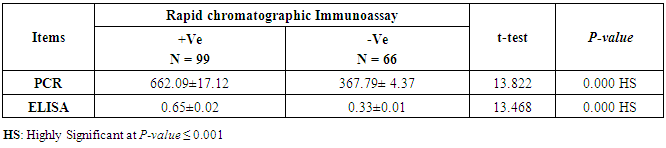
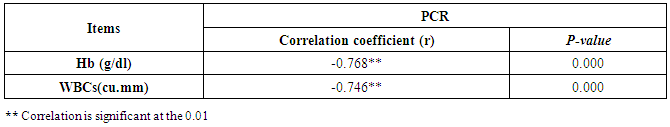
- CMV infection was much higher than other intrauterine infections like congenital toxoplasmosis (1%) and congenital rubella infection (2.7%) [20]. Primary CMV infection in an individual can be detected by demonstration of CMV specific IgM antibody [21]. CMV infection may be increased indirect effects caused by many risk factors which assumed to be associated with CMV among patients’ pregnant woman (PW) with repeated abortion. The evidence for association of CMV with these conditions is based on epidemiologic findings that show an increased risk for the infection.From the obtained results, qPCR was more efficient in detection of CMV as (96%), than ELISA (80%), while rapid chromatographic immunoassay test was (60%). The rapid test CMV card is a rapid chromatographic immunoassay for the qualitative detection of CMV antigen in the sample to aid in the diagnosis of CMV infection (IgG, IgM) [22]. As well as, many previous studies showed that, an IgG avidity assay could be used in combination with an IgM ELISA for monitoring pregnant women for primary CMV Infection [5, 23]. Diagnosis of primary infection during pregnancy is a major task of the diagnostic virology laboratory. The diagnosis of primary infection is documented the appearance of virus-specific IgG in the serum of a pregnant woman who was previously seronegative. IgM detection in a pregnant woman is likely to be a reliable marker of primary infection; as it can reveal various clinical situations as related with the acute phase of primary infection, the convalescent phase of primary infection or persistence of IgM antibody [5, 23]. Enzyme-linked immunosorbent assays (ELISAs) have been more widely used in both the indirect and the capture ELISA format, with either labeled antigen or antibody. The indirect ELISA shows the following potential sources of error when performed on whole serum [24]. The mothers of who were positive IgM antibodies although all were positive for IgG antibodies, indicating primary infection in the past or reactivation/reinfection with a different strain of CMV in early pregnancy [25].Our results also confirmed data of previous studies from Egypt and other Middle Eastern countries [26-29]. In a study from Iran by Bagheri et al. [26], the majority of pregnant women (72.1%) were positive for CMV IgG, and 2.5% were positive for CMV IgM. In Gaza strip, Palestine [30], the anti-CMV IgM was 6% among pregnant females, whereas in Turkey, the positivity for anti-CMV IgG antibody was 97.3%, while 1% were positive for anti-CMV IgM [31]. CMV total IgG antibodies were found in 92.1% in Saudi Arabia [27].As well as, real-time PCR was a rapid, sensitive and useful technique for active diagnosis disease and monitoring response to therapy [32]. From the current study, PCR and ELISA levels showed a highly significant increase in village life style patients when compared with city life style (P-value=0.000). The statistical highly significant difference was detected between the studied groups regarding age (years), time of abortion and number of delivery when compared with the use of corticosteroids (P-value=0.000). The highly significant increase of patients means that use of corticosteroids than means of patients don’t use. On the other hand, PCR and ELISA levels showed a highly significant decrease in the use of corticosteroids patients when compared with don’t use of corticosteroids (P-value=0.000). As well as, our results showed that, PCR and ELISA levels showed a highly significant decrease in blood transfusion patients when compared with don’t blood transfusion (P-value=0.000). Also, the obtained results showed statistically highly significant difference was detected between the studied groups blood transfusion regarding age (years) when compared with don’t blood transfusion as well as, time of abortion, number of abortion and number of delivery (P-value=0.000). A statistically significant positive correlation between PCR, age patients, number of abortion, Hb, WBCs, ELISA. While there was a negative correlation between PCR and time of abortion, Rapid chromatographic Immunoassay, number of delivery, use of corticosteroids, blood transfusion, H. pylori and life style. These trends of results are in consistency with Khudhur et al. [33], they detected CMV in 151 (70%) of the 214 abortion women by IgG and IgM antibody. According to the age of patients, no difference was seen in results. Also, Pouria et al. [34], Munro et al. [35] and Arabpour et al. [36] got same results, while Shams et al. [37] noticed that, there is a difference between results according to patients age. In a study by Al-Kazaz et al. [38], CMV detection by ELISA found 46 positive to IgG out of 46 and 40 positive to IgM out of 46. Ramadhan and Jihad [39] reported that miscarriage women were the highest percentage of seropositive to CMV for IgG (40%) and (25%) for IgM out of 40 samples. Yasir [40] showed higher of IgG positive at age (27-32), and also Sotoodeh [41] showed 94% of positive IgG at age (25-34). Previous studies showed that the primary CMV infection during pregnancy appears to be a much greater risk factor for congenital CMV infection than recurrent infections. Among infants born to women with primary infections during pregnancy, 32.3% had congenital CMV infection. In contrast, among infants born to pregnant women with recurrent CMV infections, 1.4% had congenital infection. The studies of pregnant women with possible primary infection all used IgM as a marker for primary infection in IgG-positive women. It has since been shown that women who have been IgG positive for a long time can be positive for IgM [42, 43], suggesting that IgM is also a marker for recurrent infection.Risk factors for CMV infection have been correlated with the socioeconomic status within a community [44, 45]. Other studies showed that elderly persons seem to be well protected against symptomatic CMV disease due to the accumulation of CD28 effector cytotoxic T lymphocytes. This is a characteristic feature of all age groups but is most pronounced in elderly persons [46].In consistency with our findings, Shams et al. [37] found that from 327 pregnant women 54 were anti CMV positive. Anti-CMV was detected in 94% of the women who had abortion history. Edmunds et al. [47] conducted their study on 130 (aborted women, suspected men, and pregnant women) could detect positive IgM to 70 patient and positive IgG to 97 patients. The prevalence rates of human cytomegalovirus IgM and IgG in non-pregnant women have been reported to be 1% and 84% respectively, and 2.5% and 90% in pregnant women [48]. Khalf et al. [49] detected positive IgM to CMV in 15.7% of 108 pregnant women. Also, Mahdi et al. [50] reported that, an increase in seropositive CMV-IgG in relation to abortion and infection. As a consequence of placental infection, CMV impairs cytotrophoblast differentiation and invasiveness; this could explain early abortion occurring in women with primary infection. In this study, all samples with positive results for IgG could be attributable to many factors one of these factors, the majority of women of childbearing age are seropositive for CMV and that they contract the infection either through prenatal or postnatal transmission during early childhood and these trends of results are in matching with findings by Ali et al. [51]. Another factor is the poor of living conditions and hygienic practices, which facilitates that, CMV is easily transmitted by many routes; sexual and non-sexual routes [52]. Risk factors for CMV infection have been correlated with the socioeconomic status within the community [45]. In addition, the ELISA result seropositivity of all the cases mention before, showed there is a significant association between recurrent abortion, premature delivery and intrauterine death and cytomegalovirus infections. A hypothesis for the probable role of geographical influence upon CMV seroprevalence might be the route of infection. In rural areas, saliva is probably the main route through which the virus is transmitted postnatally. This is likely to be the route through which the virus is transmitted early in life amongst infants and young children due to poor sanitation [53].On the other hand, in urban areas sexual transmission seems to be the major route of infection later in life during childbearing age. Another factor that may contribute to human CMV infection prevalence is geographical distribution [54-56]. Moreover, primary infections rate was higher throughout urban areas [57]. In the present study, CMV specific IgM antibody was detected in (15.7%) of all pregnant women tested indicating the substantial prevalence of infection in the local population. Our result is higher than the result reported by Alwan [48]; (46.6%), this difference may be due to small sample size for their study.Also, the results obtained by the current study was in a positive relationship with the results of Ione et al. [58]. In regards with geographical distribution, our study exhibited that, the prevalence of seropositivity for CMV was higher than western Europe, America, and Australia [35]. However, our result is higher than the result of other studies that reported CMV specific IgM antibody in (12.9%) of pregnant women with complication [59].As well as, our findings support the association between H. pylori and CMV infection. It is difficult to understand the exact mechanism for H. pylori activation in pregnancy. Previous studies that investigated this relationship [60, 61]. Lanciers et al. [62] have also found that pregnancy may be associated with increased susceptibility to H. pylori infection. Possible explanations for this could be an altered gastric pH as a result of altered fluid dynamics in pregnancy, which may result in the activation of a latent H. pylori infection; also, altered humoral and cell-mediated immunity during pregnancy, as well as changes in the various classes of antibodies during different gestational periods, could lead to H. pylori activation. All these hypotheses might explain increased seropositivity for H. pylori during pregnancy and may associate with other infection including CMV.
5. Conclusions
- Our findings indicate that, CMV infection is a common in our local population and rt-PCR is the best diagnostic tool for the diagnose as well as, ELISA which considered as a sufficient method in most cases. this high seroprevalence reflects the low hygienic standards and practices on our part. Also, CMV can lead to substantial damage to the fetus and as the damage done in utero cannot be reverted, control of intrauterine CMV infection is important. Hence, prevention of CMV infection, is essential by a screening of pregnant women, to reduce the fatal outcome of the pregnancy occurring due to the CMV infection. So, we recommend pregnant women should be attentive to disease prevention guidelines on personal hygiene during pregnancy, especially hand washing after handling diapers or oral secretions. But we recommend high risk pregnant women for example mothers that use corticosteroid or after blood transfusion and rural communities should be screened for CMV serological test during pregnancy. All pregnant women, especially with bad obstetric history, should be screened for the presence of CMV infections as well as, screening against other maternal infection to exclude any congenital infection such as (S TO R CH) is mandatory. These risk factors suggest that interventions might be most efficient if they are targeted toward certain groups.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML