-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2016; 5(2): 34-45
doi:10.5923/j.ijvmb.20160502.02

Molecular and Biological Characterization of a Nucleopolyhedrovirus Isolate (Egy-SlNPV) from Spodoptera littoralis in Egypt
Yasmein E. Ahmed1, Shimaa M. Desoky2, Marwa M. El-Sabagh1, Ahmed R. Sofy3
1Plant Protection Institute, Agric. Research Centre, Dokki, Giza, Egypt
2Botany and Microbiology Department, Faculty of Science, Suez University, Egypt
3Botany and Microbiology Department, Faculty of Science, Al-Azhar University, 11884 Nasr City, Cairo, Egypt
Correspondence to: Ahmed R. Sofy, Botany and Microbiology Department, Faculty of Science, Al-Azhar University, 11884 Nasr City, Cairo, Egypt.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The Egyptian cotton leafworm, Spodopteralittoralis (Boisd.) is an important pest causes extensive economic losses in many cultivated crops all over the world. The viruses within the family Baculoviridae are specific pathogens of insects. The nucleopolyhedroviruses (NPVs), 1 of the 2 genera of this family, have been isolated from many insect orders, primarily from the lepidopterans. In our study, NPV was isolated from the larvae of S.littoralis in Egypt. The viral occlusion bodies (VOBs) were detected using a light microscope by staining thin smear of infected larvae and drop of VOBs with Giemsa stain which appear polyhedral and negative stain particles. The toxicity of this isolate against S.littoralis also studied, by testing five different concentrations from the occlusion bodies produced by this virus isolate against 2nd and 4th instar larvae of S.littoralis. and the results indicated that the 2nd instars larvae was more susceptible to all concentrations than the 4th instars larvae, evidenced by the very low LC25, LC50, LC75 and LC90 values. The LC50 calculated from toxicity experiment used in treatment 2nd and 4th insatr larvae to study the biological properties of this isolate. For the molecular identification of the virus, polyhedra (occlusion bodies [OBs]) were isolated from insects and total DNA extraction was performed. Polyhedrin gene of S. littoralisNPV was partially amplified by PCR and its nucleotide sequence was determined. An Open Reading Frame (ORF) of 138 nucleotides was detected. Multiple sequence alignment and phylogenetic analysis were performed to compare Egy-SlNPV-polh gene with other 55 polh genes sequences from various nucleopolyhydroviruses (NPVs) and with 15 granulin genes from granuloviruses (GVs) available in GenBank. Neighbour-joining phylogeny of the nucleotide sequence and deduced amino acid sequences, clearly showed that our Egy-SlNPV isolate belongs to subgroup II-B NPVs, which is defined to include the spodopteran taxa SpliNPV, SpltNPV and SpteNPV. In case of the nucleotide sequence, the Egy-SlNPV isolate showed the close relationship with SpliNPV isolates, wihle in case of deduced amino acid sequence showed the close relationship with SpliNPV, SpltNPV and SpteNPV solates. The change of nucleotide of Egy-SlNPV at position number 76 resulted in the difference at one amino acid position with group II-B NPVs isolates (SpliNPV, SpltNPV and SpteNPV) viz. at position number 26 [methionine (M) is present in place of valine (V) a hydrophobic amino acid]. The polh gene nucleotide sequence of the Egyptian isolate (Egy-SlNPV) was registered under GenBank accession number KY072799. This study suggested the Egy-SlNPV isolate may be useful as a potential biocontrol agent in program of integrated pest management (IPM).
Keywords: Spodoptera littoralis, Toxicity, Molecular phylogeny, Nucleopolyhedrovirus, SpliNPV
Cite this paper: Yasmein E. Ahmed, Shimaa M. Desoky, Marwa M. El-Sabagh, Ahmed R. Sofy, Molecular and Biological Characterization of a Nucleopolyhedrovirus Isolate (Egy-SlNPV) from Spodoptera littoralis in Egypt, International Journal of Virology and Molecular Biology, Vol. 5 No. 2, 2016, pp. 34-45. doi: 10.5923/j.ijvmb.20160502.02.
Article Outline
1. Introduction
- The Egyptian cotton leafworm, Spodoptera littoralis (Boisd) is one of the most notorious and destructive phytophagous insect pests in Egypt, not only to cotton, but also to other field crops and vegetables [1]. These caterpillars are very polyphagous, causing important economic losses in both greenhouses and open field in a broad range of ornamental, industrial and vegetable crops. The problems and hazards that have arisen as a result of using conventional insecticides were incentives for the search of alternative control agents. Microbial control agents are a primary means of biological control of insect pests. The use of microbial control agents, targets for a particular pest species. The entomopathogens that have mostly been used in biological control include representatives of bacteria, fungi, viruses and nematodes [2, 3].Baculoviruses are considered to be the largest and most broadly studied insect viruses. They are infectious for arthropods, particularly insects of the order Lepidoptera. Baculovirus infections have been reported in over 600 insect species of the orders Hymenoptera, Diptera, Coleoptera, Neuroptera, Trichoptera, and Thysanura, as well as in the Crustaceae order Decapoda [4]. It is believed that baculoviruses are safe and selective bioinsecticides, restricted to invertebrates. The family is divided into two genera, nucleopolyhedrovirus (NPV) and granulovirus (GV). Both genera have virions occluded in a protein matrix, the occlusion body (OBs), in the NPV called polyhedra range in size from 1 to 15 µm in diameter. The NPV has been well investigated because of its potential for use as an insect pest control agent. Polyhedrin is the most abundant protein of polyhedra and the baculoviuses produce it at very high levels in the late phase of infection; polyhedrin is a protein of about 245 to 250 amino acids. The polyhedrin has been characterized in many different viruses and shown to be highly conserved. The level of similarity observed in the polyhedrin gene and protein sequence has been used for the construction of baculoviruses phylogenetic trees [5]. Therefore, the investigation of polyhedrin gene structure is important to identify the NPV. So, the main objective of the present work is to study the toxicity and biological characterization of an Egyptian isolate to be used as effective and safe biological agent against S. littoralis, also obtain a sequenced highly conserved DNA fragment from our NPV isolate to be used in an easy, fast and economic prospective system for virus detection and may be useful as a potential biocontrol agent in program of integrated pest management (IPM).
2. Materials and Methods
2.1. Virus Isolate
- The original virus isolate was obtained from diseased Spodoptera littoralis larvae collected from cotton of Agriculture Research Center, Qaha, Al-Fayom and El-Salhya. The larvae showed baculovirus infection symptoms were brought to our laboratory and examined to confirm the presence of virus by light microscope with Giemsa staining according to Mustafa, et al. [6], in which a thin smear of infected worm tissue was prepared on a glass slide and dried in air. The smear was immersed for 1-2 min in Giemsa, rinsed under running tap water for 5-10 sec then the smear was stained for two hours in 10% Giemsa stain (10g of Giemsa dissolved in 100ml distilled water), the dye was rinsed off in running tap water for 5-10 sec and allowed to dry in the air, then examined under a light microscope to detect the Occlusion Bodies (OBs). After the examination the diseased larvae kept at -20°C until the purification of OBs (polyhedra).
2.2. Virus Propagation
- The propagation of the virus isolate was performed by inoculation of the 3rd instar larvae of S. littoralis with SlNPV Egyptian isolate which collected from the field and tested by light microscopy by surface contamination of the artificial diet. The inoculated larvae were observed daily to identify the NPV infected ones based on the sign and symptoms of disease. The tissues of dead larvae were examined as soon as possible with the naked eye and tissue smears under light microscopy as mentioned above.
2.3. Viral Occlusion Bodies (VOBs) Purification
- The method of OBs purification was done according to Sudhakar et al. [7] with some modification. The individually dead larvae showing symptoms of NPV were transferred to a micro centrifuge tube and homogenized in 300 µl of distilled water. The homogenates were filtered through a chees cloth. The filtrate was subjected to sucrose layer (60% wt/vol) and centrifuged for 30 min at 10.000 rpm. The band was formed on top of the layer containing OBs was collected and again subjected to sucrose layer (40% wt/vol) and centrifuged at 10.000 rpm for 30 min. The band at the bottom of the gradient containing the OBs was collected and washed with distilled water. All the above steps were carried out at 4°C. Pure OBs were suspended in distilled water and stored at –20°C. For evaluation of OBs purification method, slide of OBs was stained with Giemsa stain as mentioned above and examined under light microscopy. The number of OBs was counted by using Neubaur Hemocytometer to determine the concentration of OBs/ml. Five concentrations were prepared from the viral OBs mother suspension by serial dilution to be used in bioassay experiment.
2.4. Insect Rearing and Biological Studies
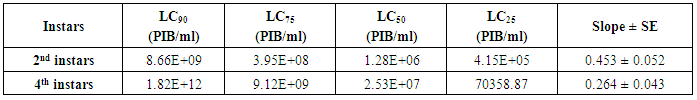
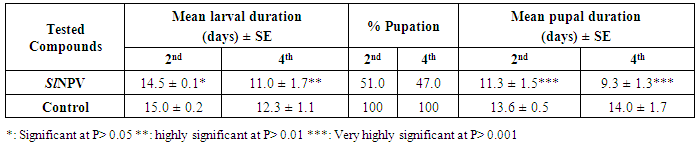
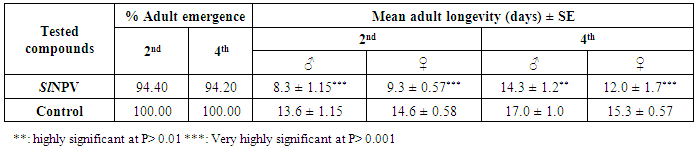
- The cotton leafworm, S. littoralis (neonate) from the laboratory of Insect Pathogen Production unit of Plant Protection Research Institute, Agriculture Research Center were reared for three generations under highly controlled conditions to avoid any insecticide contamination. Larvae fed on artificial diet described by Shorey and Hale [8]. The insect culture was maintained at 30°C, 70-80% relative humidity and a 16 h photoperiod [9, 10].The toxicity experiment was carried out using diet surface treatment procedure [11]. 2nd and 4th instars larvae of S. littoralis were starved for 16-20h at 30°C [12], then transfer to cups with contaminated artificial diet with 20µl of (1x1010, 1x109, 1x108, 1x107, and 1x106 PIBs/ml) concentrations individually. After 2 days of feeding, the diet had become unpalatable, and the larvae were transferred to clean cups of diet and observed daily. Bioassays with 30 larvae per virus concentration plus 30 larvae, as control were replicated 3 times. The experiment was conducted at a constant temperature 30°C. Larval mortality was recorded at 2 day intervals during 10 days. The mortality percentages were corrected according to Abbott’s formula [13]. Toxicity was presented graphically as log/probit regression lines, and LC25, LC50, and LC90 values as well as the slope of the probit lines were calculated [14]. LC50 was used in treatment the 2nd and 4th instar larvae to study the following biological parameters: larval and pupal duration of each instar, percentage of pupation, percentage of adult emergence, longevity of moths and the fecundity and fertility of eggs/female. A control was set comprising a similar number of untreated moths.
2.5. Molecular Characterization of Nucleopolyhedrovirus Egyptian Isolate (Egy-SlNPV)
2.5.1. Extraction of Viral DNA
- The viral DNA was purified from the OBs according to the guanidium thiocyanate DNA extraction method described by Hammond et al. [15] with slight modifications. Viral OBs were treated with the extraction solution (0.5 M guanidium thicyanate and 0.1 M EDTA) and 7.5 M ammonium acetate, the mixture was made to stand on ice for 10 min and then mixed with 1:1 phenol: chloroform, this was then centrifuged at 14.000rpm for 10min in a micro centrifuge. The upper aqueous phase was recovered carefully and placed in a new eppendorf tube. 0.5 volume isopropanol was added, the DNA was precipitated and collected after centrifugation at 14.000rpm for 20min, the DNA was washed with 70% ethanol, leave to dry for 30min then resuspended in 20 µl distilled water.
2.5.2. Primer Design and PCR Amplification of Egy-SlNPV Polyhedrin Gene
- The universal primer sets used in PCR reaction were designed for partial Egy-SlNPV-polh gene and synthesized in Operon, (Qiagene Co.), where the forward primer was 5′-GCCGAATACACCACTTCGTT-3′ and the reverse primer was 5′-TGTCGCTCGTGTTCAAGATC-3′. PCR amplification was performed according to Saiki et al. [16] with minor modification. Total reaction volume was 50 µl which contained 5 µl of 10x reaction buffer (600 mM tris HCL pH 8.3, 250 mM KCL, 1% triton X100, 100 mM B-mercaptoethanol, 2 mM MgCl2), 5 µl of 1mM dNTps, 2.5 µl Taq DNA polymerase, 1 µl of each primer and 1 µl of template DNA. The amplification was carried out using UNO-Thermoblock system from Biometra. Hard denaturation of the DNA was performed at 94°C for 1min followed by 35 cycles of amplification with denaturation at 94°C for 30 sec, annealing at 57°C for 30sec and extension at 72°C for 1min. A single tailing cycle of long extension at 72°C for 5min was carried out in order to ensure flush ends on the DNA molecules. The PCR product of the polyhedrin gene was determined by electrophorasis onto 1% agarose gel containing ethidium bromide (20 µg/ml) in 1x TAE buffer to examine the actual size of the PCR product. Agarose gel electropholrasis was performed in DNA mini electropholrasis sub-cell. Eight µl of PCR product and 8 µl of standard DNA marker (100 bp ladders) was mixed with 2 µl of 6x gel loading buffer. The PCR product was visualized on a UV transilluminator (wave length = 254 nm) and photographed by the camera.
2.5.3. Sequencing of Egy-SlNPV-polh Gene
- DNA fragment was purified from agarose gel using QIAquick Gel Extraction Kit (Qiagen, Cat. No. 28704). The nucleotide sequence of the PCR product of polh gene carried out (through Sigma Company in Egypt) by dideoxy sequencing using Capillary ABI PRISMTM BigDye® Terminator v3.1 Cycle Sequencing Ready Reaction Kit with AmpliTaq DNA Polymerase, FS and performed on automated DNA Sequencer. Data were analysed using FinchTVTM version 1.4.0 software of sequencing analysis. The polh gene nucleotide sequence of the Egyptian isolate nucleopolyhedrovirus from Spodoptera littoralis (Egy-SlNPV) was registered under GenBank accession number KY072799.
2.5.4. Sequence Alignments and Phylogenetic Analyses
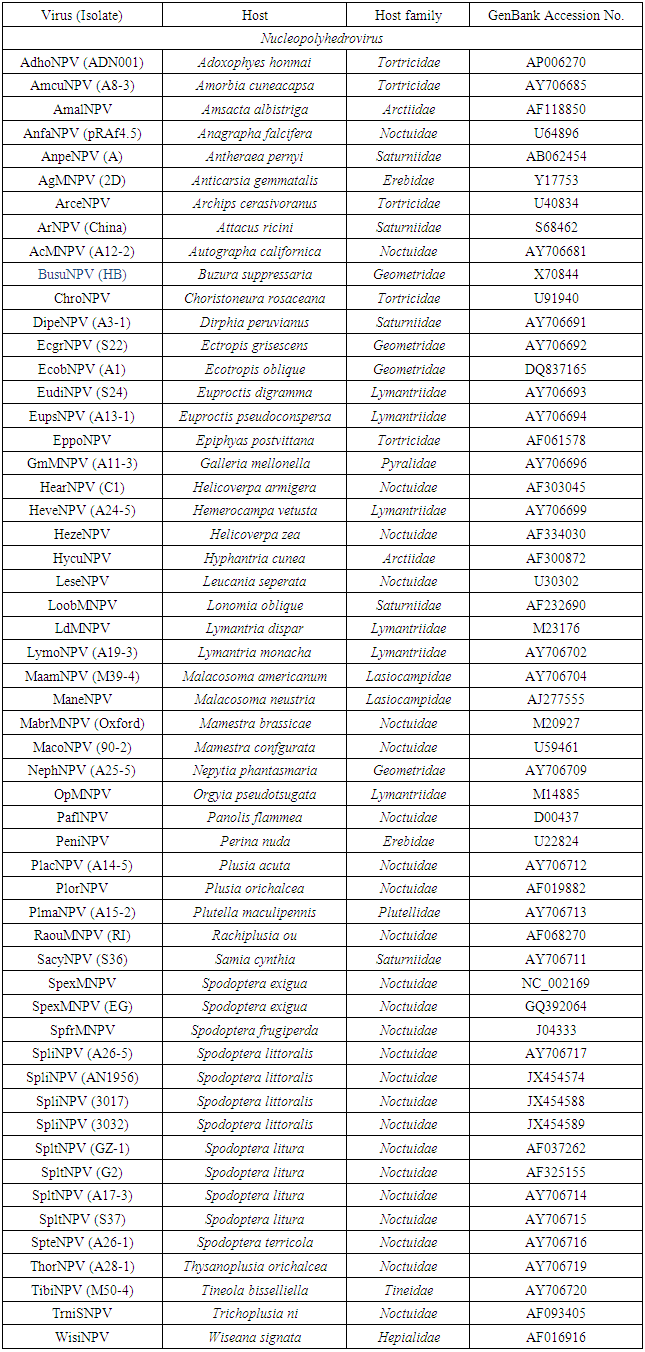
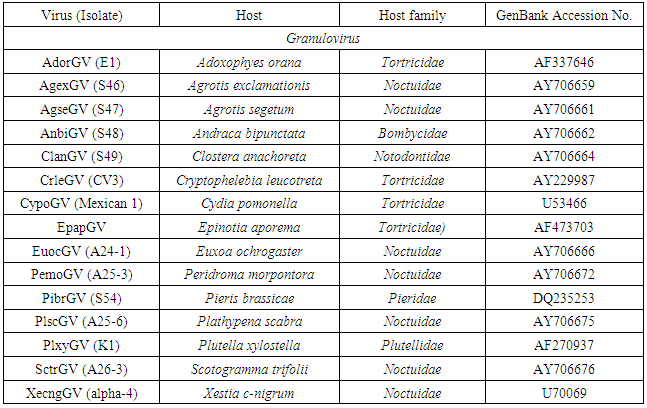
- Multiple alignments of partial Egy-SlNPV-polh gene with other corresponding polh/gran gene sequences from 55 NPVs and 15 GVs (Table 1) sequences were performed using BioEdit software (Ver.7.2.5) and ClustalW (Ver.1.74) program [17]. The virus names, abbreviations and accession numbers were listed in table (1). Neighbour-joining phylogenetic trees (1000 bootstrap replicates) were inferred from the nucleotide and deduced amino acid sequences alignments by using MEGA 4.0 software [18]. Distance matrices from aligned nucleotide and deduced amino acid sequences were determined by using the Kimura 2-parameter model [19] and the JTT matrix-based model [20], respectively, for correction of superimposed substitutions with the Molecular Evolutionary Genetics Analysis (MEGA) software (Ver. 4.0) [18]. The rate variation among sites was modeled with a gamma distribution (shape parameter = 5).
|
|
3. Results
3.1. Sample Collection and Light Microscopy Examination
- The virus isolate was obtained from diseased Spodoptera littoralis larvae (showed symptoms of viral infection) collected from cotton of Agriculture Research Center, Qaha, Al-Fayom and El-Salhya. Viral symptoms of infected S. littoralis which collected from the field can be summarized in: slow motion larvae (Fig. 1-A), cuticle showing red color (Fig. 1-B), hanging larvae (Fig. 1-C) and liquefied larval body (Fig. 1-D). The virus was propagated in a S. littoralis laboratory colony, purified and kept at -80°C for further studies. The viral occlusion bodies (VOBs) were detected using a light microscope by staining thin smear of infected larvae and drop of VOBs with Giemsa stain which appear polyhedral and negative stain particles (Fig. 2).
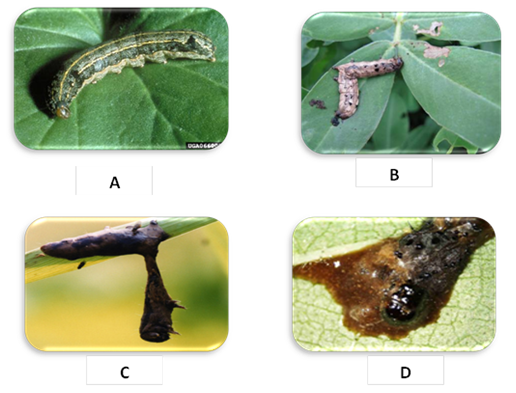 | Figure 1. Viral symptoms of infected Spodoptera littoralis showing slow motion larvae (A), red color cuticle (B), hanging larvae (C) and liquefied larval body (D) |
 | Figure 2. Viral occlusion bodies polyhedra of SlNPV under light microscopy |
3.2. Biological Studies of Nucleopolyhedrovirus Egyptian Isolate (Egy-SlNPV) against Spodoptera Littoralis
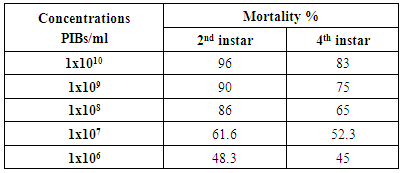
- The results of bioassay studies of Egy-SlNPV against S. littoralis are presented in table (2). The data showed that, the mortality percentage in the two tested instar larvae increased with increasing the concentrations and time elapsed post treatment. The NPV concentration 1x1010 PIB/ml ingested by larvae produces approximately 96% mortality percentage at 10 days post inoculation for S. littoralis 2nd instar larvae and 83% of the same concentration for 4th instar larvae. The remaining concentrations produce mortality percentages ranged from 90 to 48% and 75 to 45% of the two tested instars larvae used, respectively.
|
|
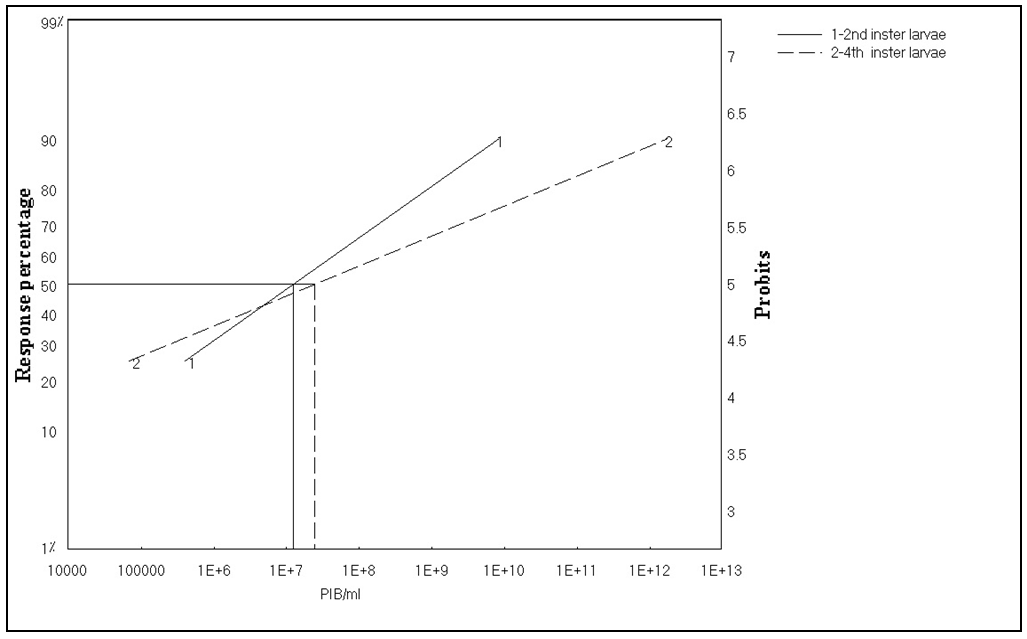 | Figure 3. Toxicity lines of SlNPV on 2nd and 4th instars larvae of Spodoptera littoralis |
|
|
|
3.3. Molecular Characterization of the Egy-SlNPV-polh Gene
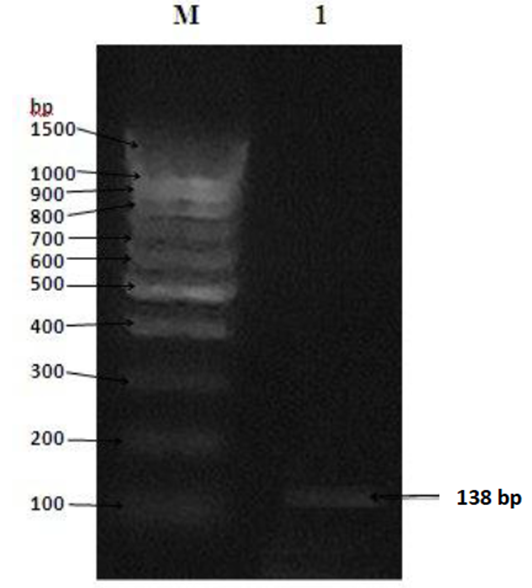
- The partial codons sequence of the polyhedrin (polh) gene was amplified using specific PCR primers. Electrophoresis analysis of the PCR product showed a single fragment at ~138 bp of the test naturally infected S. littoralis (Fig. 4). The sequence obtained for the polh gene was 138 bp long, representing 0.55/3 of the complete polh open reading frame (ORF), which comprised 1425 bases of nucleotides potentially coding for 46 amino acids.
3.3.1. Nucleotide and Deduced Amino Acid Sequences Analysis
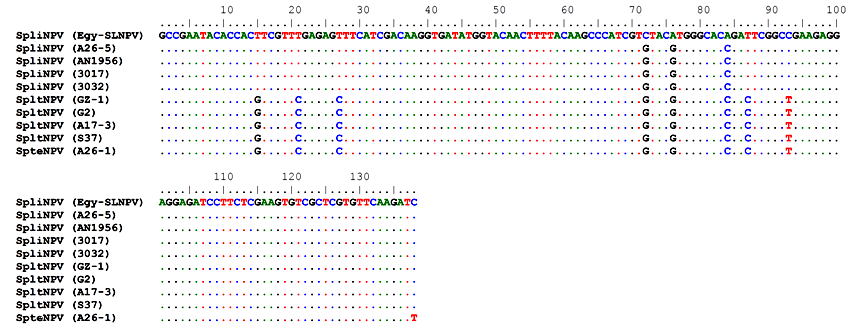
- Multiple sequence alignment and phylogenetic analysis were performed to compare Egy-SlNPV-polh gene with other 55 polh genes sequences from various nucleopolyhydroviruses (NPVs) and with 15 granulin genes from granuloviruses (GVs) available in GenBank (Table 1).The pairwise similarity of the nucleotide and deduced amino acid sequences of the polh/gran gene of nucleopolyhedrovirus Egyptian isolate (Egy-SlNPV) obtained from naturally infected S. littoralis showed 71.0–97.8% and 76.1-97.8, respectively, homology with reported isolates of NPVs in NCBI GenBank as compared to 58.0-68.1% and 52.2-58.7% with nucleotide and amino acid sequences of the out group member (GVs), respectively. The nucleotide sequence of the polh gene of Egy-SlNPV showed maximum homology (97.8%) with the four nucleopolyhedrovirus isolates (SpliNPV) from Spodoptera littoralis (A26-5, AN1956, 3017 and 3032 under Accession Nos. AY706717, JX454574, JX454588 and JX454589, respectively), and 94.2% with nucleopolyhedrovirus isolates (SpltNPV) from S. litura (GZ-1, G2, A17-3 and S37 under Accession Nos. AF037262, AF325155, AY706714 and AY706715, respectively). On the other hand, the deduced amino acid sequence of the polh gene of Egy-SlNPV showed maximum homology (97.8%) with 9 nucleopolyhedrovirus isolates [4 SpliNPV, 4 SpltNPV and 1 isolate from S. terricola (SpteNPV A26-1 under Accession No. AY706716)].
3.3.2. Phylogenetic Analysis
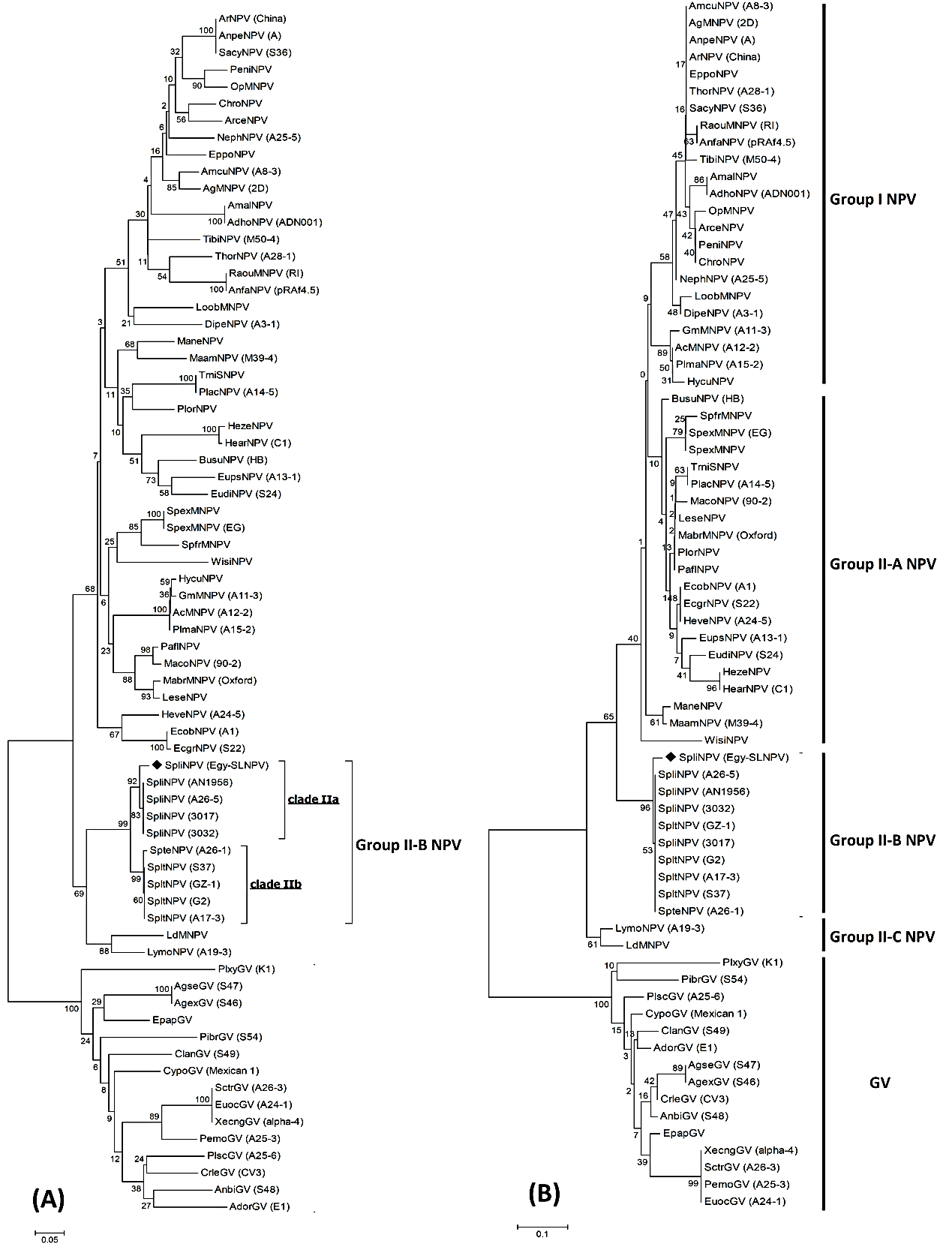
- Phylogenetic analyses of the partial nucleotide and deduced amino acid sequences of Egy-SlNPV-polh gene was used to consider whether the Egy-SlNPV is consistent with viral infective evolution, a conservative test of whether they are likely to represent nucleopolyhedrovirus infect Spodoptera littoralis. Neighbour-joining phylogeny of the Egy-SlNPV (Fig. 5) indicated the presence of two distinct NPVs groups (I & II), where group II NPVs includes three subgroups (II-A, II-B & II-C), almost all of which are supported by high bootstrap scores in the phylogeny of the polh gene alignment (Fig. 5-A & B).Within the subgroup II-B NPVs in a phylogenetic tree of the nucleotide sequences, two monophyletic clades were identified (Fig. 5-A). The first clade (IIa) included the five SpliNPV isolates (Egy-SlNPV, A26-5, AN1956, 3017 and 3032). The second clade (IIb) included the SpteNPV isolate (A26-1) and four isolates of SpltNPV (GZ-1, G2, A17-3 and S37). On the other hand, the subgroup II-B NPVs in a phylogenetic tree of the deduced amino acid sequences contains one monophyletic clade (Fig. 5-B). It was included 5 SpliNPV isolates, 4 SpltNPV isolates and SpteNPV isolate. From the phylogenetic analyses of the nucleotide and deduced amino acid sequences, that clearly showed that our Egy-SlNPV isolate belongs to subgroup II-B NPVs. On the other hand, granuloviruses (GVs) was positioned as a separated group (out group member).
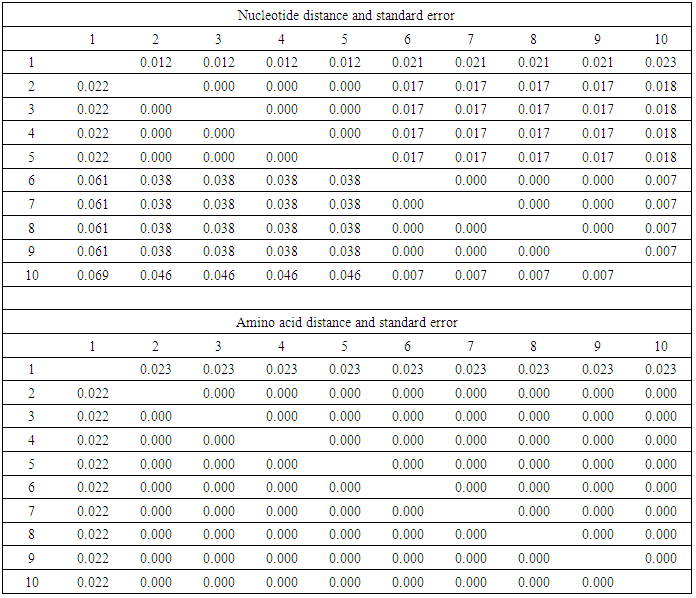
3.3.3. Evolutionary Divergence between Egy-SlNPV Isolate and Subgroup II-B NPVs Isolates
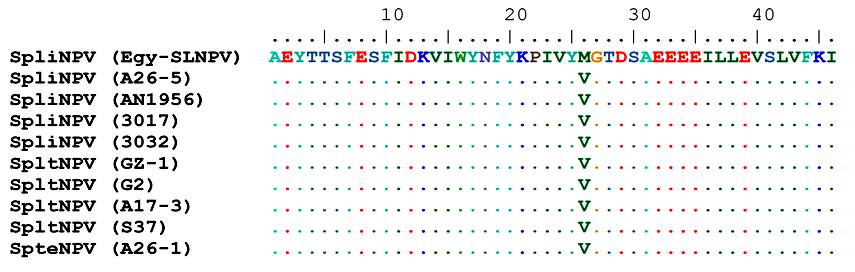
- The polh gene nucleotide and deduced amino acid sequences alignment were analyzed to identify amino acid mutations that occurred on multiple branches basal to the defined subgroup II-B NPVs (Figs. 6 & 7). All of these sequences were multiple aligned using the ClustalW program with minor manual adjustments, where the number of base and amino acid substitutions per site from between sequences are shown in table (7). Standard error estimate (s) are shown above the diagonal. The nucleotide sequence analyses were conducted using the Kimura 2-parameter (K-2-P) model where the rate nucleotide sequence variation among sites was modeled with a gamma distribution (shape parameter = 5), while the amino acid sequence analyses were conducted using the JTT matrix-based model with a gamma distribution (shape parameter = 5).The analysis involved 10 nucleotides and amino acid sequences of subgroup II-B NPVs, including Egy-SlNPV isolate. All ambiguous positions were removed for each sequence pair. There were a total of 138 and 46 positions in the final dataset for nucleotide and amino acid sequences, respectively. A total of 9 and 1 variable sites were found in subgroup II-B NPVs nucleotide and amino acids, respectively, including the gaps where 5 and 0 were parsimoniously informative nucleotide and amino acids, respectively. As well as, 4 and 1 were singleton sites, respectively (Figs. 6 & 7). The differences in nucleotides of Egy-SlNPV with other subgroup II-B NPVs isolates were also present at various nucleotide positions due to replacement of either purines with pyrimidines (one place), purine to purine (one place) or pyrimidines with purines (at one place) viz. at positions number 72 (G is replaced by C), 76 (G is replaced by A) and 84 (C is replaced by A), respectively. The change of nucleotide at position number 76 resulted in the difference at one amino acid position with group II-B NPVs isolates viz. at position number 26 [methionine (M) is present in place of valine (V) a hydrophobic amino acid] (Fig. 7).
|
4. Discussion
- The Egyptian cotton leafworm, Spodoptera littoralis Boisd. (Lepidoptera: Noctuidae) is one of the most notorious and destructive phytophagous insect pests in Egypt, not only to cotton, but also to other field crops and vegetables [1]. The new nature baculovirus isolates with better insecticidal characteristics is safer and has not the risks of releasing genetically engineered product in nature. Seufi [21] reported that baculoviruses have been isolated from Upper as well as Lower Egypt, in which the cultivation was washed by chemical insecticides, it would be expected to find more and more isolates in virgin regions where no insecticides were used. So, the main objective of the present work is to study the biological and molecular characterization of an Egyptian isolate to be used as effective and safe biological agent against S. littoralis, where it become may be useful as a potential biocontrol agent in program of integrated pest management (IPM).The virus isolate was obtained from diseased Spodoptera littoralis larvae exhibited viral-like symptoms collected from cotton of Agriculture Research Center, Qaha, Al-Fayom and El-Salhya. Viral symptoms of infected S. littoralis which collected from the field are slow motion larvae, cuticle showing red color, hanging larvae and liquefied larval body, where the similar results were reported by Toprak et al. [22]. The viral occlusion bodies (VOBs) of the nucleopolyhedrovirus Egyptian isolate (Egy-SlNPV) were detected using a light microscope by staining thin smear of infected larvae and drop of VOBs with Giemsa stain which appear polyhedral and negative stain particles. The occlusion bodies of NPVs are specifically identified as polyhedral, where baculoviruses occlude their virions in large, proteinaceous occlusion bodies which help the virus to remain viable outside the host for years [23].The infectivity of Egy-SlNPV against S. littoralis larvae revealed that 2nd instar larvae were more sensitive than 4th instar larvae. The sensitivity of young instar was reported by Gomezet et al. [24]. Different susceptibility levels of S. littoralis larvae to NPV were observed by several authors [21, 25, 26]. Such difference in susceptibility may be due to the difference in larval age, the number of virions contained in occlusion bodies and the feeding habit of the insect [26, 27]. The results were agreeable to that of Stairs [28], who found that susceptibility decreased markedly as larvae of Malacosoma disstria grew older. In parallel, Duan and Otvos [29] reported that mortality was higher when younger larvae of Choristonura fumiferana were used.The LC50 values and un parallel toxicity lines also proved the susceptibility of younger instar than older one, and this agree with finding of Whitlock [30] in Heliothis armigera and differ from those of Gitanjali et al. [31] and Monobrullah and Nagata [32]. In the later, it was concluded that the responses of larvae of all instars were similar since the line of different instars were parallel.On the basis of biological assays there is a great reduction of all biological parameters studied and these results have agreed with those of Abdel-Salam et al. [33] who treated S. littoralis larvae with Viroset (SlNPV); and with those of Yasmein [34], who treated larvae of S. littoralis with Littovir (SlNPV).The Egy-SlNPV isolate infected S. littoralis was detected and identified by PCR using polyhedrin gene primers. The polyhedrin gene proved to be suitable for the development of the generic amplification technique, the polyhedrin gene is highly conserved between NPVs, making it the preferred choice for phylogeny studies [35]. Although the length and molecular mass of polyhedrin are somewhat different, depending on the species, the high similarity could build the phylogenetic trees on the basis of polyhedrin sequences. The full length of the polyhedrin gene from lepidopteran NPVs ranged from 483 bp to 747 bp, where in case of polyhedrin gene from Spodoptera sp. NPVs, its full length ranges from 510 bp to 747 bp [36].The partial polyhedrin (polh) gene sequence (138 bp) of the PCR-amplified fragment of the nucleopolyhedrovirus Egyptian isolate (Egy-SlNPV) was done to determine the sequence information and variability with other corresponding partial 55 polh genes sequences from various nucleopolyhydroviruses (NPVs) and 15 granulin genes from granuloviruses (GVs) available in GenBank. Consequently, these partial sequences allowed the identification and classification of the newly analyzed Egy-SlNPV isolate. Neighbour-joining phylogeny of the partial polh gene sequence of Egy-SlNPV indicated the presence of two distinct NPVs groups (I & II), where NPVs taxa from Lepidoptera form a closely related clade, while the branch lengths of the GV clade are generally longer. These results in agreement with Zanotto et al. [37] and Cowan et al. [38], who reported that based on the reconstruction of the phylogenetic relationships of polyhedrin genes and on diferential relative rate of evolution, classed NPVs into groups I and II. Also, from the phylogenetic analyses of the nucleotide and deduced amino acid sequences, that clearly showed that our Egy-SlNPV isolate belongs to subgroup II-B NPVs, where group II NPVs includes three subgroups (II-A, II-B & II-C). Subgroup II-B is defined to include the the spodopteran taxa SpliNPV, SpltNPV and SpteNPV, where these results are in agreement with Bulach et al. [35].
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML