-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2015; 4(2): 23-36
doi:10.5923/j.ijvmb.20150402.02

First Molecular Characterization of Strawberry Vein Banding Virus Naturally Spread in Mixed Infection with Fruit Phyllody Phytoplasma in Egypt
Ahmed R. Sofy
Department of Botany and Microbiology, Faculty of Science, Al-Azhar University, Cairo, Egypt
Correspondence to: Ahmed R. Sofy , Department of Botany and Microbiology, Faculty of Science, Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of this work study the molecular characterization of coat protein (CP) gene of Strawberry vein banding virus (SVBV) mixed infection with fruit phyllody phytoplasma according to suspected symptoms of vein banding, mottling, yellowing, vein necrosis, leaf crinkling, deformation, phyllody fruits and stunting were recorded on fresh strawberry (Fragaria × ananassa Duchesne), at Qalyubia Governorate, during the growing season of 2014. The SVBV and phytoplasma were detected in naturally infected strawberry plants by DBIA and Diene’s stain, respectively. PCR assay with SVBV-CP gene specific primers yielded an amplicon of full CP gene 1425 bp from symptomatic strawberry with vein banding leaves, and fruit phyllody symptoms. While, no amplification was observed in the test sample with fruit phyllody symptoms only. The CP gene variability between the Egyptian isolate and the European isolate (Acc. No. X97304); was 0.77 % (11 nt substitutions in a 1425 nt long region) lead to the variability of the deduced amino acids was 1.3% (6 aa substitutions in a 474 aa long region). Similar to the variation of the three North American isolates (Acc. Nos. AY605662, AY605663, and AY605664) with 0.7%, where 9 nt substitutions in a 1382 nt long region, lead to variability 0.9% (4 aa substitutions in a 468 aa long region). So, neighbour-joining phylogeny of the nucleotide and deduced amino acid sequence of the SVBV-EG isolate showed the close relationship with European, and North American isolates. There are identified two molecular markers (SNPs) in SVBV-EG isolate where the identified A to T change of nucleotide at position number 47 produces a His to Leu amino acid change at position number 16, thus replacing a positively charged residue with a hydrophobic one. Also, the identified C to A change of nucleotide at position number 1021 produces a Gln to Lys amino acid change at position number 341, thus replacing a polar residue with a positively charged residue. The CP gene nucleotide sequence of the Egyptian isolate was registered under GenBank accession number KU366260. This is the first sequence of the SVBV-CP gene from Egypt registered on GenBank. The phytoplasma aetiology was firstly detected through a combination of sectioned plant tissues by the application of Dienes' stain, observed as dark blue areas in the phloem region, using a light microscope, and then confirmed by a nested PCR assay with phytoplasma-specific universal primer pairs (P1/P7 and R16F2n/R16R2). Hence, this study suggested the mixed infection of SVBV and fruit phyllody phytoplasma with strawberry.
Keywords: Strawberry, SVBV, DBIA, Sequence, Molecular variability, Phytoplasma, Dienes' stain, Nested PCR
Cite this paper: Ahmed R. Sofy , First Molecular Characterization of Strawberry Vein Banding Virus Naturally Spread in Mixed Infection with Fruit Phyllody Phytoplasma in Egypt, International Journal of Virology and Molecular Biology, Vol. 4 No. 2, 2015, pp. 23-36. doi: 10.5923/j.ijvmb.20150402.02.
1. Introduction
- Commercial strawberry (Fragaria × ananassa Duchesne), which originated in Europe around 1750, is a hybrid between F. virginiana Duchesne from North America and F. chiloensis (L.) Duchesne from South America. It is one of the preferable and important horticultural crops in Egypt for fresh consumption, food processing and for exportation and growing in Egypt as a vegetable crop [1, 2].Strawberry vein banding caulimovirus (SVBV, genus: Caulimovirus, family: Caulimoviridae) is one of the most economically important viruses of strawberry in the majority of production areas [3].It has a double-stranded DNA genome of approximately 8 kb encapsidated in icosahedral particles of approximately 45 nm diameter. The virus has seven open reading frames where ORF IV encodes the viral coat protein of approximately 471 amino acids [3, 4]. It is transmitted by aphids (Chaetosiphon spp.) in a semipersistent manner and by grafting [5]. It was reported to reduce runner production, yield, and fruit quality in the United States in commercial fields [6]. The characteristic symptoms of vein banding are seen as chlorotic banding along the primary and secondary veins. The detection of SVBV is rather difficult, however. No commercially produced antibodies are available and thus no serological detection is possible. The only certified method for the moment is the time-consuming bioassay on indicator plants [7]. But, biological studies of SVBV and other strawberry-infecting viruses are obstructed by the properties of the host plant where sap transmission of SVBV has been unsuccessful [4]. So, in Egypt, the occurrence of SVBV has been reported from strawberry material when detected in field samples using PCR by Ragab et al. [8]. Diverse group of phytoplasmas has been reported worldwide on strawberry. Phyllody of strawberry (Fragaria x ananassa Duch.) fruit is exemplified by the reversion of floral reproductive members, generally around achenes, into vegetative structures [9]. Strawberry fruit phyllody symptoms have been associated with the presence of phytoplasmas belonging to the aster yellows group [eastern aster yellows (16Sr I, subgroup A), western aster yellows (16Sr I, subgroup B)], and strawberry green petal (clover phyllody, 16Sr I, subgroup C) [9], “Candidatus Phytoplasma australiense” (16Sr XII, subgroup B) [10], and Mexican periwinkle virescence phytoplasma (16Sr XIII, subgroup B) [11].Jomantiene et al. [12] found four phytoplasmas associated with phyllody of strawberry fruit: strawberry multicipita (SM) phytoplasma phylogenetically classified in 16S rRNA (16Sr) group VI, subgroup B), STRAWB2 phytoplasma (16Sr I, subgroup K), clover yellow edge phytoplasma (16Sr III, subgroup A), and a new Group III phytoplasma.In the present study, we examined strawberry plants that produced fruit exhibiting phyllody, and vein banding leaves suggestive of virus and phytoplasmas-like agents under tropical conditions. The aim of the present investigation was to record the association of pathogen(s) with the strawberry disease syndrome for the first time in the Egypt. Due to the previous absence of any sequencing data of SVBV in Egypt, we report the CP nucleotide sequence of SVBV and compare it with the sequences of previously SVBV in GenBank.
2. Materials and Methods
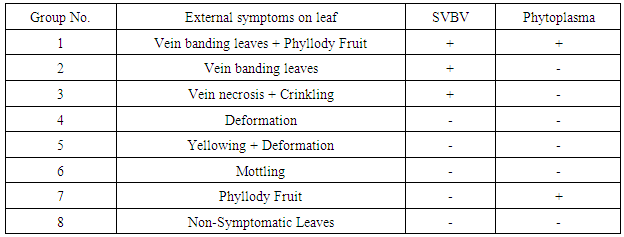
- Suspected symptomatic plant samples of fresh strawberry (Fragaria × ananassa Duchesne) showing viral-like and phytoplasma symptoms were divided into seven groups as well as the non-symptomatic group (Table 1) were collected from the Qalyubia Governorate, Egypt during the growing season of 2014 and stored at -20°C for further analysis. The Strawberry vein banding virus (SVBV) and phytoplasma were detected in the collected samples by DBIA and Diene’s stain, respectively.SVBV detection by Dot Blot Immunoassay (DBIA)The leaf samples were serologically tested for the presence of Strawberry vein banding virus using dot blot immunoassay (DBIA). The chemicals and antisera specific for viruses were kindly provided from Faculty of Agriculture, Ain Shams University, Egypt. Dot blot immunoassay was done as that described by Lin et al. [13] as follows: Nitrocellulose membranes, 0.45 µM pore size, were marked with a lead pencil into squares of 1x1 cm. The infected strawberry plants were ground in phosphate buffer pH 9.5 (1:10 W/V) as well as healthy plants as a negative control 0.5 µl was applied in each square for both healthy and virus infected samples. The membranes were washed three times with PBS-Tween at 5 minutes interval. The membranes were placed in the blocking solution (1% bovine serum albumin + 2% non fat dried milk in PBS-Tween), and incubated for one hour at room temperature followed by washing three times with PBS-Tween at 5 minutes interval. The virus specific antisera diluted in PBS (1:500) were added and then incubated for one hour at room temperature with gentle shaking followed by washing three times with PBS-Tween at 5 minutes interval. The goat antirabbit immunoglobulin - alkaline phosphatase for IgG-SVBV dilution 1:1000 were added to conjugate buffer (PBS-T + 2% PVP + 0.2% ovalbumin) and incubated for one hour at room temperature. Washing three times with PBS-Tween at 5 minutes interval was done. The substrate solution (Nitro Blue Tetrazol m and 5-bromo-4-chloro-3-indoly (phosphatase) were added and incubated for 5 minutes. After the color has appeared, membranes were rinsed quickly with H2O then air-dried.Phytoplasma detection by Dienes’ stainThe samples of strawberry (Fragaria × ananassa Duchesne) appeared virus-like symptoms were tested against phytoplasma where it was detected in the leafy bracts on phylloid fruits and the other samples in the leaves as well as healthy, ones. Leaves petioles were dissected into approximately 1-2 mm sections, using scalpe containing phloem tissue. The prepared sections were transferred onto 70% ethanol, then stained using Dienes’ stain as described by Deeley et al. [14], and examined immediately for the presence of the phytoplasma by the light microscope (400X).Molecular characterization of Strawberry vein banding virusExtraction of total DNAThe total DNA was extracted from naturally infected strawberry plant leaves (Fragaria × ananassa Duchesne) as well as the healthy ones, using the standard assay developed by Dellaporta et al. [15].Primers and PCRThe primer sets used in PCR reaction were synthesized for SVBV coat protein gene in Operon, (Qiagene Co.), were designed from the ORF IV on the complete sequence of GenBank Acc. No. NC_001725 according to Mráz et al. [16]. Forward primer was 5´1890-ATGGTAAGCAGAAGAGAAAGAC-3' and reverse primer was 3´3311-CTCCAGATCTTCTGAGTC -5´. The PCR (25µl) was performed using 1μl of DNA, 1.25 U Taq DNA Polymerase, 200μM of each dNTP, 2.5μl 10X Dream Taq Buffer, 25pmol of each primer, 2.5mM MgCl2 and 15.25μl of sterile water. PCR tubes containing DNA, extracted from healthy strawberry plants, were used as negative controls. PCR was designed as 94°C for 3min, followed by 35 cycles, 94°C for 1 min, 50°C for 1min, 72°C for 2min, and final extension at 72°C for10 minutes. The PCR amplicon was confirmed by gel electrophoresis of the expected size in 1.2% agarose gel in 1X TAE and stained with ethidium bromide revealing an amplified DNA fragment.Sequencing of SVBV coat protein geneDNA fragment was purified from agarose gel using the gel slicing and melting methods described by Wieslander [17]. The nucleotide sequence of the PCR product of coat protein gene carried out (through Sigma Company in Egypt) by dideoxy sequencing using Capillary ABI PRISMTM BigDye® Terminator v3.1 Cycle Sequencing Ready Reaction Kit with AmpliTaq DNA Polymerase, FS and performed on automated DNA Sequencer. Data were analysed using FinchTVTM version 1.4.0 software of sequencing analysis. The CP gene nucleotide sequence of the Egyptian isolate was registered under GenBank accession number KU366260.Sequence alignments and phylogenetic analysesMultiple alignments of sequences were performed using BioEdit software (Ver.7.2.5) and ClustalW (Ver.1.74) program [18]. The nucleotides and amino acids distances were estimated considering alignment gaps and using the Jukes and Cantor's method [19], and the p-distance method, respectively, for correction of superimposed substitutions with the Molecular Evolutionary Genetics Analysis (MEGA) software (Ver. 4.0) [20]. Phylogenetic relationships among SVBV sequence isolates were evaluated using Neighbour Joining (NJ) implemented through MEGA 4.0 software, and bootstrap analysis (1000 replicates) was performed to assess the reliability of the constructed phylogenetic tree. To compare variability of SVBV-EG isolate with European isolate, and American isolates vs. Chinese isolates, entropy values (Hx) along the 474 amino acids of the coat protein gene were calculated and plotted using the Entropy plot tool of BioEdit. The entropy value is a measure for nucleotide or amino acid variation at a given position in aligned sequences; it varies from 0 (i.e. no variation) to 1.06 (i.e. all the possible 20 amino acids or a gap occur in equal frequency).Identification of strawberry phylloid fruits phytoplasmaNested PCR for detection of phytoplasmaThe extracted total DNA was used in nested PCR assays for detection of phytoplasma. Two pairs of universal primers, P1/P7 and R16F2n/R16R2 were used to amplify the 16S rRNA of the phytoplasma in nested PCR [21]. The primer sets P1/P7 was used in the first step PCR-for the amplification of 1.8 kb product of 16S rDNA gene, while the primer pair R16F2n, R16R2 was used to amplify a 1.2 kb fragment of 16S rDNA gene in the second step nested-PCR as described by [22]. To run the first step PCR; 25μl PCR mixture contained 1μl target DNA, 25 pmol of each primer; 200μM of each dNTP; 1X polymerase reaction buffer; 2.5mM MgCl2; 1.25 U of Taq DNA Polymerase and sterile water to a final volume of 25μl. The DNA amplification was started with a denaturation step at 94°C for 2 min followed by 35 cycles consisting of denaturation at 94°C for 30 sec, annealing at 55°C for 1 min, and primer extension at 72°C for 1.5 min. A final extension step was added for 10 min 72°C.One μl of DNA amplified in the first step PCR was used at 1:10 dilution as a template for the second step, nested, PCR. The nested PCR was started with a denaturation step at 94°C for 2 min followed by 35 cycles consisting of denaturation at 94°C for 30 sec, annealing for 2 min at 50°C, and primer extension at 72°C for 3 min. A final extension step was added for 10 min 72°C. The PCR amplicon was confirmed by gel electrophoresis of the expected size in 1.2% agarose gel in 1X TAE and stained with ethidium bromide revealing an amplified DNA fragment.
3. Results
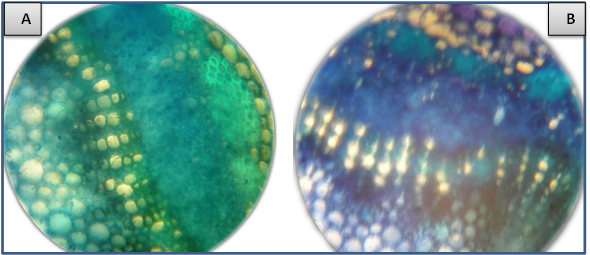
- SymptomatologyDuring the growing season of 2014 a survey of strawberry fields at Qalyubia Governorate, Egypt, viral and phytoplasma-like symptoms were observed. Since, vein banding, mottling, yellowing, vein necrosis, leaf crinkling, deformation, phyllody fruits and stunting were common among infected plants (Fig. 1). These symptoms were divided into seven groups as well as the non-symptomatic group (Table 1). Three naturally SVBV infected strawberry plants were detected serologically using DBIA (Fig. 2 & Table 1). Also, two naturally phytoplasma infected strawberry plants were detected by the application of Dienes’ stain, using a light microscope (Fig. 3 & Table 1).Hand thin leaf petiole sections of the mixed strawberry infection have a complete ring of xylem surrounded by a ring of phloem. The external phloem is separated from the xylem by a very narrow but distinct band of cambium. In all sections of phytoplasma fruit phyllody strawberry treated with Dienes’ stain, however, the xylem was colored bright turquoise blue and cells in the cortex (phloem) stained pale purplish blue. Moreover, the phloem of samples without phyllody symptoms and healthy petiole sections remained unstained but phloem of samples with phyllody symptoms petiole sections contained many regularly distributed areas that stained a distinct blue (Fig. 2).
|
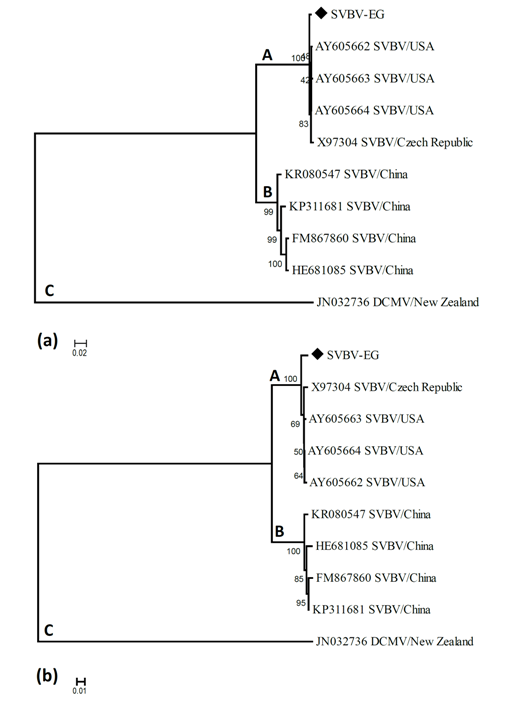 | Figure 5. Neighbour-joining tree of SVBV-EG strain and SVBV isolates available in GenBank based on based on CP gene nucleotide sequence (A) and CP gene amino acid sequence (B) alignment. The numbers represent bootstrap percentage values based on 1000 replicates. The optimal tree with the sum of branch length = 1.04273047 for nucleotide sequence and 0.75244057 for protein sequence are shown. The letters indicate the different SVBV lineages discussed in the main text |
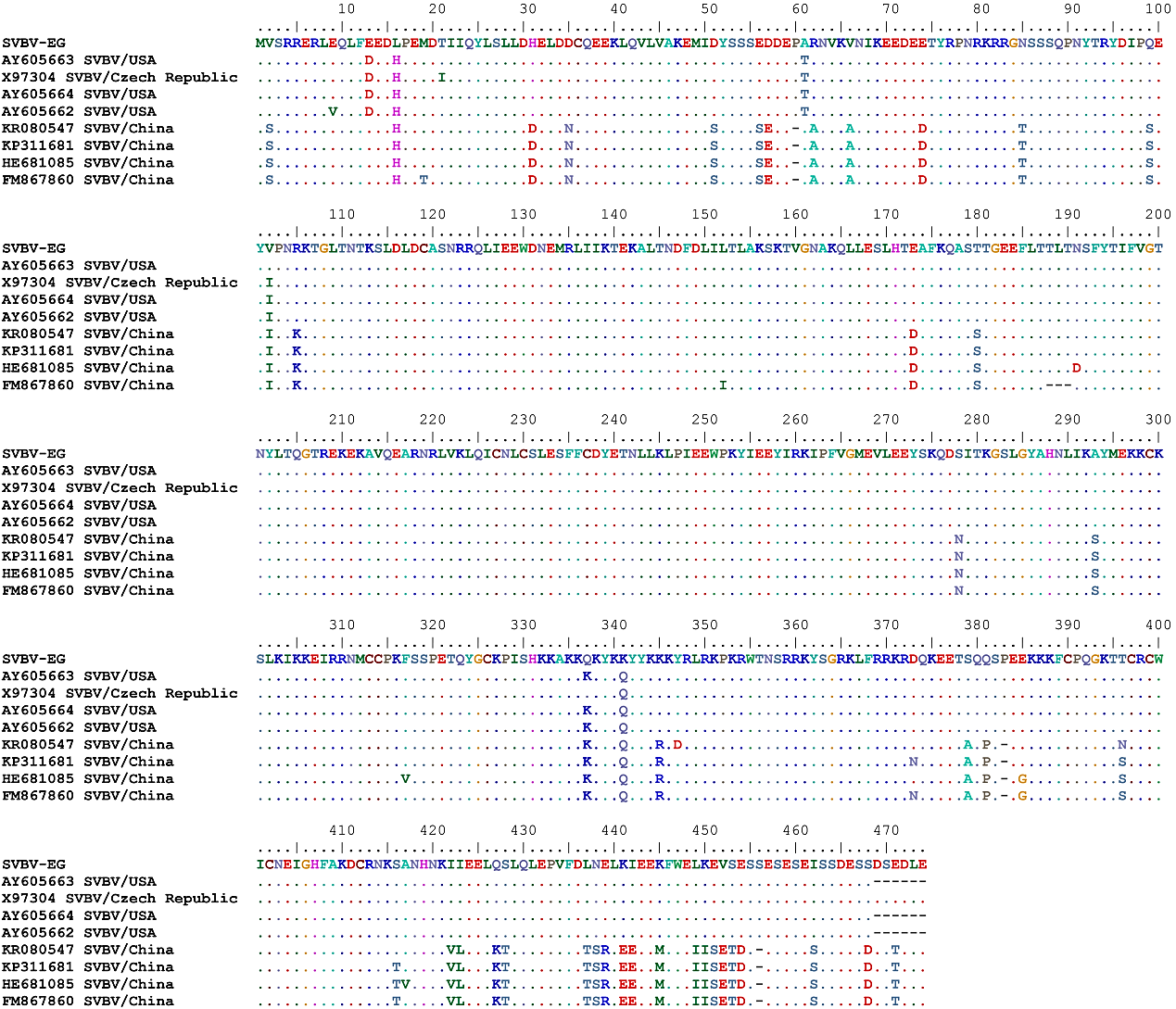 | Figure 6. Multiple amino acid sequence alignment of the complete CP gene of the studied SVBV-EG isolate with the corresponding amino acid sequence of SVBV isolates available in GenBank |
 | Figure 7. Nucleotides diversity of SVBV-EG-CP with 8 SVBV isolates showing the characteristic changes variable (V), parsimoniously informative nucleotide (Pi) and singleton (S) sites |
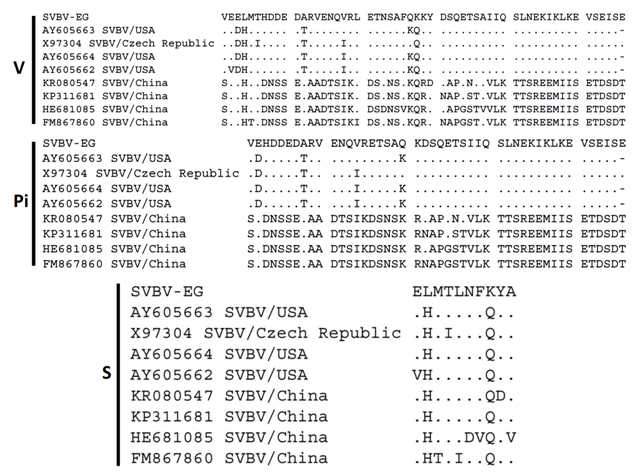 | Figure 8. Protein amino acids diversity of SVBV-EG strain with 8 SVBV isolates showing the characteristic changes variable (V), parsimoniously informative nucleotide (Pi) and singleton (S) sites |
|
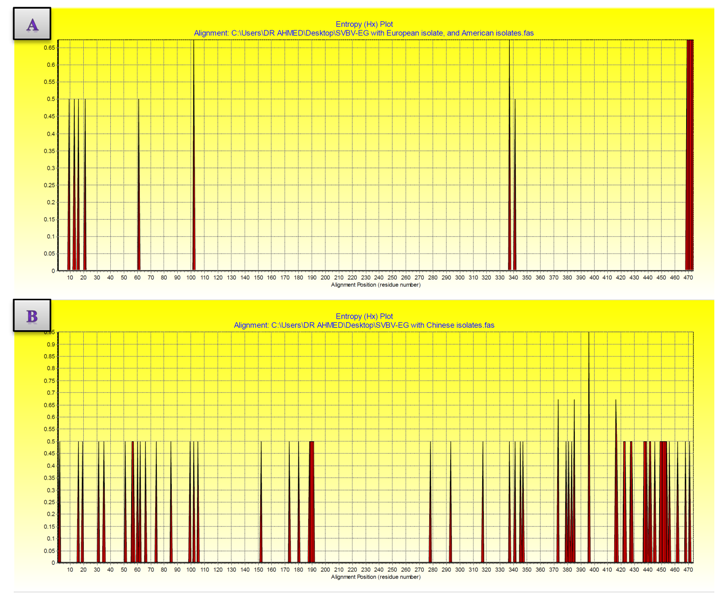 | Figure 9. Variation along the coat protein of SVBV-EG isolate with European isolate, and American isolates vs. Chinese isolates sequences shown by entropy plots. (A) Entropy plot of SVBV-EG isolate with European isolate, and American isolates. (B) Entropy plot of SVBV-EG isolate with Chinese isolates. Entropy values (Hx) are a measure of variation at each amino acid position in the set of aligned sequences |
|
|
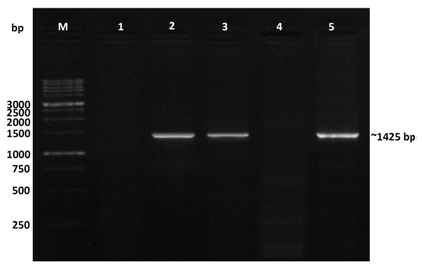
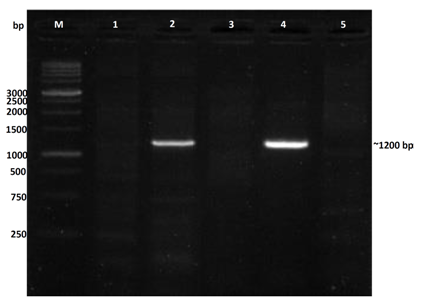 | Figure 10. Nested PCR assay with R16F2n/R16R2n primers, M = molecular weight marker (1 kb ladder); lane 1: non-symptomatic strawberry sample (healthy control); lane 2: vein banding leaves and fruit phyllody symptoms; lane 3: vein banding leaves symptoms; lane 4: strawberry plants only with fruit phyllody symptoms; lane 5: vein necrosis and crinkling symptoms |
4. Discussion
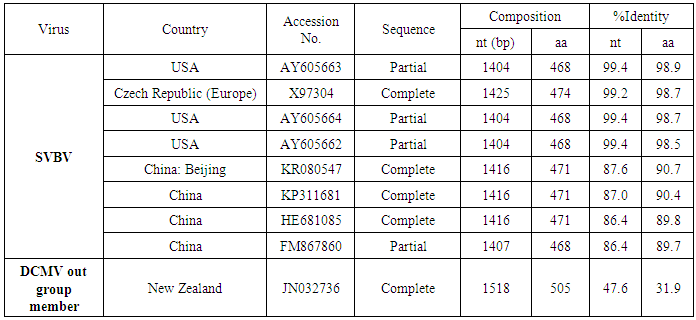
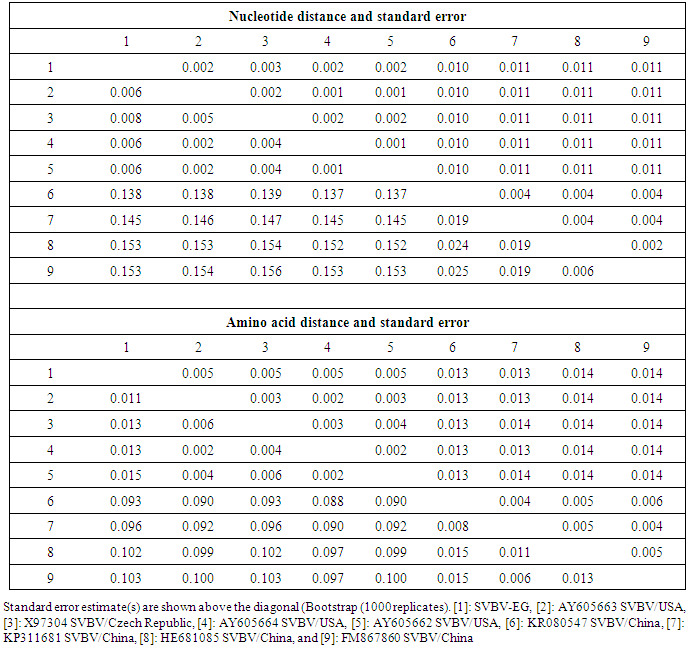
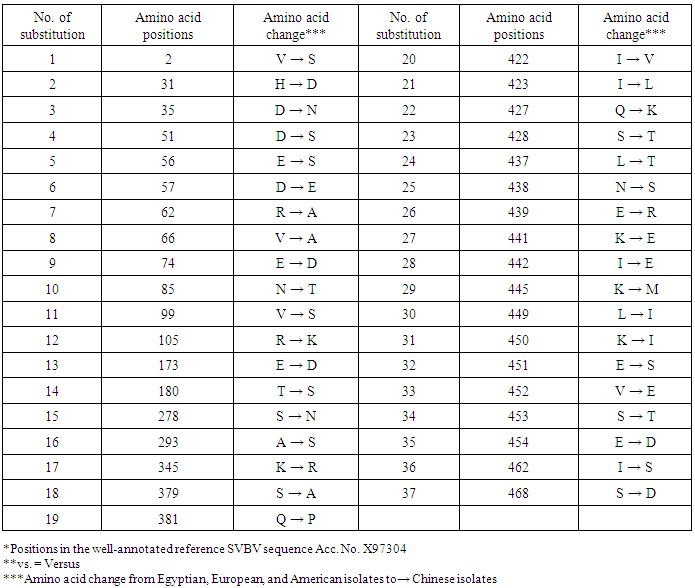
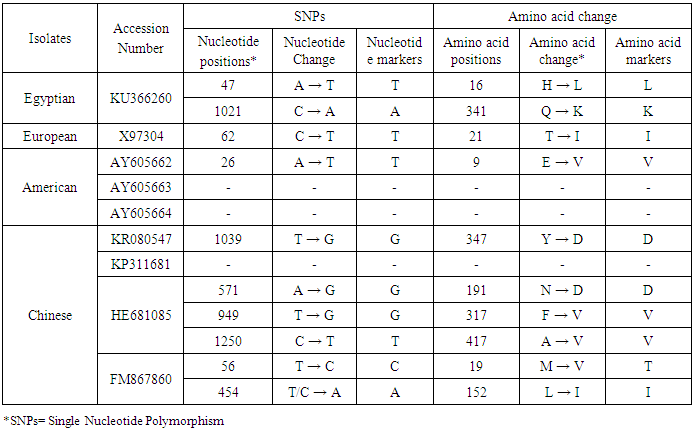
- In the present study, recorded, naturally mixed infection on fresh strawberry plants with Strawberry vein banding virus (SVBV) and Phytoplasma which distinct vein banding leaf symptoms and fruit phyllody symptoms. During 2014 the fresh strawberry plants (Fragaria × ananassa Duchesne) cultivated in open field (Qalyubia Governorate, Egypt) showing vein banding, mottling, yellowing, vein necrosis, leaf crinkling, deformation, phyllody fruits, and stunting symptoms. According to the distinct symptoms have shown that individual plants, serologically indexed for SVBV by DBIA, and indexed for phytoplasma by Dienes' stain. These plants carried SVBV, probably an indication of mixed infection with phytoplasma. Where, Phyllody fruit is exemplified by the reversion of floral reproductive members, generally around achenes, into vegetative structures. Reversion generally begins at the distal end of the receptacle and progresses toward the calyx. Strawberry fruit phyllody symptoms have been associated with the presence of phytoplasma agent [10, 11]. The strawberry plant is a vegetatively propagated perennial and, therefore, the health control of the propagation material is important for its cultivation. SVBV has been considered one of the most economically important viruses of strawberry in the majority of production areas [1, 7]. It was reported to reduce runner production, yield, and fruit quality in the United States in commercial fields [6]. Symptoms of SVBV infection vary in different strawberry species and cultivars where appear chlorotic banding or streaks of veins, necrosis of veins and interveinal area, and leaf curling in F. vesca and F. virginiana strawberry. However, in most cultivated strawberries, SVBV is symptomless [6, 23]. So, in plant production schemes it is important to use specific virus tests such as grafting onto indicator plants, DBIA, ELISA, RT-PCR or PCR for virus indexing rather than rely on visual inspections [23]. The detection of SVBV is rather difficult, however. No commercially produced antibodies are available and thus no serological detection is possible. The only certified method for the moment is the time-consuming bioassay on indicator plants [7]. But, biological studies of SVBV and other strawberry-infecting viruses are obstructed by the properties of the host plant where sap transmission of SVBV has been unsuccessful [4, 24]. Besides, no alternative host for SVBV has been identified to facilitate biological studies. Thus, procedures for obtaining purified virus in quantities needed for molecular characterization of the virus genome and virion proteins, and for producing specific antibodies, are still limited by the difficulties associated with strawberry tissues [25]. Also, molecular methods based on the detection of the specific viral nucleic acids represent an important improvement of the situation [7].The goals of this paper to record the association of pathogen (s) with the strawberry disease syndrome for the first time in the Egypt. Where, the fruit phyllody symptoms of strawberry associated with phytoplasma not recorded before in Egypt but, Ragab et al. [8] was detected SVBV in Egypt at the first time in field samples using PCR only. So, also, the aim of this work study the molecular characterization of SVBV Egyptian isolate (SVBV-EG) through a coat protein (CP) gene sequence to obtain a more complete picture of the variability of SVBV-EG with the nucleotide sequences already available in GenBank at the first time in Egypt.Strawberry vein banding virus (SVBV) was detected through PCR assay with SVBV coat protein-specific primers yielded an amplicon of approximately ~1425 bp both from the sample with vein banding leaves and fruit phyllody symptoms and from the two samples with vein banding leaves symptoms; vein necrosis and crinkling symptoms. While, no amplification was observed in the test sample with fruit phyllody symptoms only. No fragment was amplified from the DNA extracted from non-symptomatic strawberry plant (healthy plant control). The results were expected as the same range of the SVBV/CP amplified from clone pSVBV-E3 [3].The full capsid protein gene sequence (1425 bp) of the PCR-amplified fragment of the SVBV-EG isolate was done to determine the sequence information and variability with other isolates available in GenBank over the world. Variability between the Egyptian isolate and the European isolate (pSVBV-E3) with the whole CP gene sequence Acc. No. X97304 [3], was 0.77 % (11 nt substitutions in a 1425 nt long region) lead to the variability of the deduced amino acids was 1.3% (6 aa substitutions in a 474 aa long region). Also, this was similar to the variation of the three North American isolates (9010, 9044 and 9093; Acc. Nos. AY605663, AY605664, and AY605662, respectively) observed by Vašková et al. [7] who found 9 nt substitutions in a 1382 nt long region (variability of 0.7 %), lead to 4 aa substitutions in a 468 aa long region (variability of 0.9%). So, Neighbour-joining phylogeny of the nucleotide and deduced amino acid sequence of the SVBV-EG isolate showed the close relationship with European isolate, and North American isolates, thereby depicting their association with each other. Also, comparison of the resulting entropy plots showed higher entropy values in SVBV-EG isolate with European isolate, and American isolates than Chinese isolates, where amino acid sequences from the Egyptian isolate, European isolate, American isolates, and Chinese isolates datasets were compared by univariable analysis.The SVBV-EG/CP (ORF IV) contains at amino acid position 399-412 the conserved zinc-finger domain with the arrangement Cx2Cx4Hx4C typical of the coat protein of all the caulimoviruses. This was similar to the European isolate [3], American isolates [7], and Chinese isolates. Strikingly, there are identified two SNPs in SVBV-EG isolate where the identified A to T change of nucleotide 47 produces a His to Leu amino acid change at position number 16, thus replacing a positively charged residue with a hydrophobic one. Also, the identified C to A change of nucleotide 1021 produces a Gln to Lys amino acid change at position number 341, thus replacing a polar residue with a positively charged residue. These SNPs consider potential markers for SVBV Egyptian isolate where it is the most important source of variability and one of the most common types of genetic variation.On the other hand, to confirm that naturally occurring SVBV in mix infection with phytoplasma, the test strawberry samples were firstly detected through a combination of sectioned plant tissues by the application of Dienes' stain, observed as dark blue areas in the phloem region, using a light microscope, and then confirmed by a nested PCR assay. The detection method applied in the present study is widely used to detect phytoplasma where nested PCR most widely used to overcome the low concentration problem of phytoplasma in infected plants. The efficiency of nested PCR clearly shows in amplifying of the direct PCR product where, the amplifications provide a simple, rapid approach to obtain rRNA that may either be sequenced directly or cloned and sorted through a recombinant genomic DNA library to find a specific gene [26] however, this technique requires more than one PCR step which increasing the chances of contamination between samples [27]. Furthermore, phytoplasma was successfully detected using nested PCR instead of direct one, because they often occur at low levels in plants, making the detection of DNA by direct PCR is unreliable may be attributed to inhibitors present in host plant tissue [10, 28, 29]. So, nested PCR with primer pair R16F2n/R16R2 yielded approximately 1200-bp amplicon in the test leafy bracts on phylloid fruits of of strawberry (Fragaria × ananassa Duchesne), while universal phytoplasma-specific primer pair P1/P7 gave no amplification in the first round of PCR assay.Jomantiene et al. [12] found four distinct phytoplasmas associated with phyllody of strawberry and suggest that fruit phyllody in strawberry may be a general symptom associated with phytoplasma infection, where all plants were assessed for phytoplasma infection by use of nested PCR primed by phytoplasma universal primer pairs R16mF2/R1 and F2n/R2 [30] or P1/P7 [31] and F2n/R2.The results represent the first knowledge about the mixed infection of SVBV and phytoplasma on strawberry in Egypt. Therefore, it would be important to evaluate the role of different epidemiological factors in natural spread of this syndrome. The resistant genotypes, management of insect vectors and alternate/collateral hosts would be the most efficient control majors for the syndrome. Based on a combination of results obtained by the PCR and sequence analysis of the virus, it was concluded that the SVBV-EG isolated from diseased strawberry plants showed the close relationship with European isolate, and North American isolates. This is the first sequence of the SVBV-CP gene from Egypt registered on GenBank.
References
| [1] | Spiegel S. and Martin R.R., 1998, Virus and viruslike diseases. Pages 62-72 in: Compendium of Strawberry Diseases. J. L. Maas, ed. American Phytopathological Society, St. Paul, MN. |
| [2] | Karesova R., Vaskova H. and Paprstein F., 2004, Investigation of occurrence and testing of aphid-borne strawberry viruses. Acta Horticulturae, 656, 63-67. |
| [3] | Petrzik K., Beneš V., Mráz I., Honetšlegrová-Fránová J., Ansorge W. and Špak J., 1998, Strawberry Vein Banding Virus—Definitive Member of the Genus Caulimovirus. Virus Genes, 16(3), 303-305. |
| [4] | Stenger D.C., Mullin R.H. and Morris T.J., 1988, Isolation, molecular cloning, and detection of Strawberry vein banding virus DNA. Phytopathology, 78, 154-159. |
| [5] | Frazier N.W. and Converse R.F., 1980, Description of Plant Viruses, CMI and AAB, Kew, Surrey, England, No. 219. |
| [6] | Dara S.K., 2015, Virus decline of strawberry in California and the role of insect vectors and associated viruses. Plant Health Progress doi:10.1094/PHPMR-15-0023. |
| [7] | Vašková D.H., Špak J. and Petrzik K., 2006, Variability in sequence of Strawberry vein banding virus. Biologia Plantarum, 50(4), 660-666. |
| [8] | Ragab M., El-Dougdoug K., Mousa S., Attia A., Sobolev I., Spiegel S., Freeman S., Zeidan M., Tzanetakis I.E. and Martin R.R., 2009, Detection of strawberry viruses in Egypt. Acta Hortic., 842, 319-322. |
| [9] | Clark M.F., 1998, Leafhopper-vectored diseases caused by phytoplasmas, p. 72-75. In: J.L. Maas (ed.), Compendium of strawberry diseases. APS Press, St. Paul, Minnesota. |
| [10] | Andersen M.T., Beever R.E., Gilman A.C., Liefting L.W., Balmori E.A., Beck D.L., Sutherland P., Bryan G., Gardner R. and Forster R., 1998, Detection of phormium yellow leaf phytoplasma in New Zealand flax (Phormium tenax) using nested PCRs. Plant Pathology, 47, 1881-96. |
| [11] | Harrison N.A., Legard D.E., DiBonito R. and Richardson P.A., 1997, Detection and differentiation of phytoplasmas associated with diseases of strawberry in Florida. Plant Dis., 81, 230. |
| [12] | Jomantiene R., Maas J.L., Dally E.L. and Davis R.E., 2002, Phytoplasmas in strawberry with fruit phyllody. Acta Hortic., 567, 635-638. |
| [13] | Lin, N.S., Hsn Y.H. and Hsu H.T., 1990, Immunological detection of plant viruses and mycoplasmalike organism by direct tissue blotting on nitrocellouse membranes. Phytopathology, 80, 824-828. |
| [14] | Deeley J., Stevens W.A. and Fox R.T.V., 1979, Use of Dienes’ stain to detect plant diseases induced by mycoplasma like organisms. Phytopathol., 69, 1169-1171. |
| [15] | Dellaporta S.L., Wood J. and Hicks J.B., 1983, Maize DNA minipreps. Maize Genet. Coop. Newsl. 57, 26-29. |
| [16] | Mráz I., Petrzik K., Šíp M. and Fránová-Honetšlegrová J., 1998, Variability in coat protein sequence homology among American and European sources of strawberry vein banding virus. Plant Dis., 82, 544-546. |
| [17] | Wieslander L., 1979, A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Analytical Biochemistry, 98, 305-309. |
| [18] | Thompson J.D., Higgins D.G. and Gibson T.J., 1994, clustalw. Improving the sensitivity of progressive multiple sequence alignment through sequencing weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res., 22, 4673-4680. |
| [19] | Jukes T.H. and Cantor C.R., 1969, Evolution of protein molecules. In: Munro HN (ed) Mammalian Protein Metabolism. New York, Academic Press, pp 21-132. |
| [20] | Tamura K., Dudley J., Nei M. and Kumar S., 2007, MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol., 24, 1596-1599. |
| [21] | Bhat A.I., Madhubala R., Hareesh P.S. and Anandaraj M., 2006, Detection and characterization of the phytoplasma associated with a phyllody disease of black pepper (Piper nigrum L.) in India. Scientia Hortic., 107, 200-204. |
| [22] | Wang K. and Hiruki C., 2001, Use of heteroduplex mobility assay for identification and differentiation of phytoplasmas in the aster yellows group and the clover proliferation group. Phytopathology, 91(6), 546-52. |
| [23] | Martin R.R. and Tzanetakis I.E., 2006, Characterization and recent advances in detection of strawberry viruses. Plant Dis., 90, 384-396. |
| [24] | Morris T.J., Mullin R.H., Sclegel D.E., Cole A. and Alosi M.C., 1980, Isolation of a caulimovirus from strawberry tissue infected with Strawberry vein banding virus. Phytopathology, 70, 156-160. |
| [25] | Mahmoudpour A., 2003, Infectivity of recombinant Strawberry vein banding virus DNA. Journal of General Virology, 84, 1377-1381. |
| [26] | Giovannoni S., 1991, The polymerase chain reaction. In: Stackebrandt E, Goodfellow M (eds) Nucleic Acids Techniques in Bacterial Systematics, pp. 177-203. John Wiley & Sons, Chichester. |
| [27] | Olmos A., Cambra M., Esteban O., Gorris M.T. and Terrada E., 1999, New device and method for capture, reverse transcription and nested PCR in a single closed tube. Nucleic Acids Research., 27, 1564-1565. |
| [28] | Cheung W.Y., Hubert N. and Landry B.S., 1993, A simple and rapid DNA microextraction method for plant, animal, and insect suitable for RAPD and other PCR analyses. Genome Research, 3, 69-70. |
| [29] | Goodwin P.H., Xue B.G., Kuske C.R. and Sears M.K., 1994, Amplification of plasmid DNA to detect plant pathogenic mycoplasmalike organisms. Annals of Applied Biology, 124, 27-36. |
| [30] | Gunderson D.E. and Lee I.M., 1996, Ultrasensitive detection of phytoplasmas by nested-PCR assays using two universal primer pairs. Phytopath. Medit., 35, 144-151. |
| [31] | Jomantiene R., Davis R.E., Dally E.L. and Maas J.L., 1998, The distinctive morphology of ‘Fragaria multicipita’ is due to phytoplasma. HortScience, 33, 1069-1072. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML