-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2015; 4(1): 12-18
doi:10.5923/j.ijvmb.20150401.03
Changes in Serum Cortisol, Thyroid Hormones and Lipid Profiles in Nigerian Men and Women on 1st and 2nd Line Antiretroviral Therapy for 52 Weeks
Ebuehi O. A. T.1, Awolola A.1, Akanmu A. S.2
1Departments of Biochemistry, College of Medicine, University of Lagos, Lagos, Nigeria
2Heamatology & Blood Transfusion, College of Medicine, University of Lagos, Lagos, Nigeria
Correspondence to: Ebuehi O. A. T., Departments of Biochemistry, College of Medicine, University of Lagos, Lagos, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
BACKGROUND: Human immunodeficiency syndrome (HIV) which affects the whole body can interfere with proper endocrine function, and hormones in turn can affect the disease progression. Cortisol suppresses many aspect of the immune response, including proliferation of lymphocytes, the activity of natural killer cells, macrophages and neutrophils, and the production of certain cytokines. Acquired immunodeficiency syndromes (AIDS) is associated with reduced thyroid hormone production.OBJECTIVE: The study is to determine the changes in cortisol, thyroid hormones and lipid profiles of Nigerian men and women on first and second line highly active antiretroviral therapy (HAART) for 52 weeks.METHODS: The serum concentration of cortisol, thyroid stimulating hormones(TSH), thyroxine(T4) and triiodothyronine(T3) and the lipids (HDL, Total cholesterol and triacyl glycerol) profiles were determined in 40 HAART positive on 1st line, 40 HAART positive on 2nd line, 40 HIV positive HAART naive and compared to 40 HIV negative controls.RESULTS: Serum concentrations of cortisol were significantly (p<0.05) increased in the patients on both HIV positive HAART experience of 1st and 2nd line and naïve subjects compared to the negative control. There was a significant decrease in the free-thyroxine (FT4) in the HIV positive group (HAART experienced and naïve) compared to the HIV negative control group. There was no significant difference in the thyrotropin (TSH) and the free triiodothyronine (FT3) in all the three HIV positive group compared to the HIV negative control group. No significant differences were observed in renal and lipid profiles in all the different groups.CONCLUSIONS: Data of the study indicate that serum levels of cortisol and thyroid hormones may be used as baseline periodic markers during antiretroviral therapy, though subtle imbalances may have a major impact on the quality of life and many people with HIV may benefit from supplementation if appropriate.
Keywords: HIV-1 infection, 1st, 2nd line HAART, Cortisol, Thyroid Hormones, Lipids, Gender
Cite this paper: Ebuehi O. A. T., Awolola A., Akanmu A. S., Changes in Serum Cortisol, Thyroid Hormones and Lipid Profiles in Nigerian Men and Women on 1st and 2nd Line Antiretroviral Therapy for 52 Weeks, International Journal of Virology and Molecular Biology, Vol. 4 No. 1, 2015, pp. 12-18. doi: 10.5923/j.ijvmb.20150401.03.
Article Outline
1. Introduction
- Highly active antiretroviral therapy (HAART), is a combination of at least three drugs for HIV-1 infection and has led to substantial reductions in morbidity and mortality, and may significantly improves prognosis of HIV in infected persons by reducing the HIV load. Increasing HAART regimens result in near complete suppression of HIV-1 replication [1], delaying progression to acquired immunodeficiency syndrome (AIDS) and reducing mortality [2, 3, 4].Hormones play a key role in maintaining homeostasis and regulating many bodily processes, from growth and metabolism to sexual function and reproduction. Over or underproduction of endocrine hormones can contribute to a wide variety of medical conditions [5]. HIV which affects the whole body can interfere with proper endocrine function, and hormones in turn can affect the disease progression [6, 7, 8]. The endocrine, nervous and immune systems are interrelated in complex ways that have not been fully understood. Cortisol suppresses many aspect of the immune response, including proliferation of lymphocytes, the activity of natural killer cells, macrophages and neutrophils; and the production of certain cytokines. High levels of cortisol are seen in individual with many types of severe, acute and chronic infections and AIDS is no exception [5]. It can be challenging to diagnose endocrine problems in people with HIV because certain symptoms may be associated with altered levels of more than one hormone. For example, fatigue and depression may be due to low level of thyroid hormones, cortisol, growth hormone or testosterone. Multiple endocrine mechanisms may interact in complex syndromes, such as wasting, lipodystrophy and other metabolic abnormalities [10, 11, 12]. Lipodystrophy has become one of the most closely watched manifestations in people with HIV. Cases of lipodystrophy have now been reported among people taking any of the four approved protease inhibitors. There have been a number of reports of lipodystrophy occurring among HIV-infected patients taking non-protease inhibitor-based HAART combinations [13, 14, 15]. Many people on HAART are also experiencing metabolic complications such as elevated glucose levels and glucose intolerance, hyperlipidemia, hypertension, along with mild elevations in cortisol secretion and hypogonadism, all of which have been associated with body composition changes in HIV-infected patients [5, 7, 12, 14]. Lipodystrophy has been found in patients with diabetes mellitus, Cushing's syndrome, and other chronic diseases. As with these other diseases, HIV-related lipodystrophy is associated with body composition changes in both men and women, glucose intolerance, hyperlipidemia, hypertension, elevations in cortisol secretion, and decreased testosterone levels [5, 12, 16, 17, 18].There is paucity of information on the changes in serum cortisol, thyroid hormones and lipid profiles of Nigerian men and women on first and second line antiretroviral therapy. Therefore, the objectives of the study are to ascertain the changes in serum cortisol, thyroid hormones and lipid profiles of Nigerian men and women on 1st and 2nd line antiretroviral therapy.
2. Materials and Methods
2.1. Study Location
- The study was carried-out at the AIDS Prevention Initiative in Nigeria (APIN) clinic of the Lagos University Teaching Hospital (LUTH), Idi-araba, Surulere, Lagos, Nigeria.
2.2. Research Subjects
- The following subjects were recruited for the study; a. Human immunodeficiency virus (HIV) infected subjects on first line highly active antiretroviral therapy (HAART) for 52 weeks;b. Human immunodeficiency virus (HIV) infected subjects on second line highly active antiretroviral therapy (HAART) for an average of 52 weeks;c. HIV infected subjects naïve to HAART;d. Non-infected human subjects
2.3. Recruitment of Subjects
- Forty subjects were recruited for each group - HAART experienced on first line therapy, HAART experienced on second line therapy, HAART naïve and non-infected human subjects were involved in this study. All subjects were at least 18 years old as at the time of this study and gender balance was taken into consideration. There was no financial or material inducement for subjects’ participation in this study.
2.4. Experimental Procedure and Sample Collection
- Eight ml whole blood samples were collected in EDTA, lithium heparin and fluoride oxalate vacutainers from volunteer HAART experienced on first and second line ART (as indicated by their patient registration/APIN identities and data, visit to clinicians, and collecting ARVs at the APIN clinic) and naïve HIV positive (as confirmed by the HIV screening done in APIN clinic using the serial testing algorithm) subjects in the research location site, while sampling for volunteer HIV negative subjects within LUTH was done after a serial screening algorithm using HIV-1 rapid test kit showed a negative result. The process of specimen collection brought some slight discomfort to the patients, but nothing was done outside the normal management of the patient. However, the benefits of this project outweigh the risk. Necessary bio-safety level facilities required for major stages in the research were put to use. In addition, laboratory standard operating procedures were strictly adhered to.
2.4.1. Determination of Serum Cortisol, FT4, FT3
- Serum cortisol determination was carried out using the method of Chopra et al., [19], a competitive enzyme immunoassay. The plates are coated with anti-cortisol antibodies. The serum reference, control or patient samples are first added to the microwell plate before adding enzyme –cortisol conjugate. Cortisol present in the sample competes with the enzyme-cortiso conjugate for binding with the anti-cortisol coated microplate to form an antigen-antibody complex. Unbound conjugate is removed by decantation. The enzyme activity in the antibody-bound fraction is inversely proportional to the native cortisol concentration. The enzyme activity is revealed by a colour change in TMB substrate solution which is measured photometrically at 450nm.
2.4.2. Determination of Thyrotropin (TSH)
- The determination of serum TSH was based on the method of Hopton and Harrop [20], an immunoenzymometric assay. Upon mixing the monoclonal biotintylated antibody, the enzyme labeled antibody and serum containing the native antigen reaction results between the native antigemand the antibodies, without competition or steric hindrance, to form a soluble sandwich complex [20]. After equilibrium is attained the antibody bound fraction is separated from the unbound antigen by decantation. The enzyme activity in the antibody-antigen bound fraction is directly proportional to the native antigen concentration.
2.4.3. Total Cholesterol
- The enzymatic method of Allain et al., [21] was used for the determination of serum total cholesterol. This is based on the determination of ∆4-cholestenone after enzymatic cleavage of the cholesterol ester by cholesterol esterase, conversion of cholesterol by cholesterol oxidase, and subsequent measurement by the Trinder reaction of the hydrogen peroxide formed [22].
2.4.4. Tri Acyl Glycerol
- The serum triacyl glycerol was determined using lipoprotein lipase (LPL) from microorganisms for the rapid and complete hydrolysis of TGs to glycerol followed by oxidation to dihydroxyacetone (DHAP) and hydrogen peroxide under the catalysis of glycerol kinase (GK) and glycerol phosphate oxidase (GPO) and also subsequent measurement by the Trinder reaction of the hydrogen peroxide formed [23].
2.4.5. HDL-Cholesterol
- The HDL-Cholesterol was assayed using the Friedewald equation by subtracting the amount of cholesterol associated with LDL and VLDL assuming a prolonged fasting state (12 – 14h). The LDL-Cholesterol was computed by subtracting Total HDL-Cholesterol from Total Cholesterol, and expressed as mmol/L [24].
2.4.6. Statistical Analysis
- Statistical analysis was carried out using SPSS version 20.0. Data were expressed as Mean ± Standard Error of the Mean (SEM). Inter-group differences were analyzed using one-way analysis of variance (ANOVA) for the comparison of three or more groups and values were considered significant at p<0.05.
3. Results and Discussion
- The mean serum cortisol of 1st and 2nd line anti retroviral therapy (ARV) patients, positive and negative controls are presented in Table 1. The mean serum concentration of HIV positive 1st and 2nd line ARV, HIV positive and negative male and female subjects are shown in Figure 1.There was a significant elevation (p>0.005) in the mean cortisol level of the human immunodeficiency virus positive male on 1st line highly active antiretroviral therapy (HIV positive 1st line HAART), human immunodeficiency virus positive male on 2nd line highly active antiretroviral therapy (HIV positive 2nd line) when compared with the human immunodeficiency virus negative / non infected person (HIV negative) (Table 1). There was a significance increase in the serum cortisol levels of human immunodeficiency virus positive/ highly active antiretroviral naïve male patient (HIV positive HAART negative) with the HIV positive male subject. The same trend was observed in the female population when compared with the mean cortisol levels of the HIV positive 1st line HAART and HIV positive 2nd line HAART with the HIV negative female subjects (Fig. 1).
|
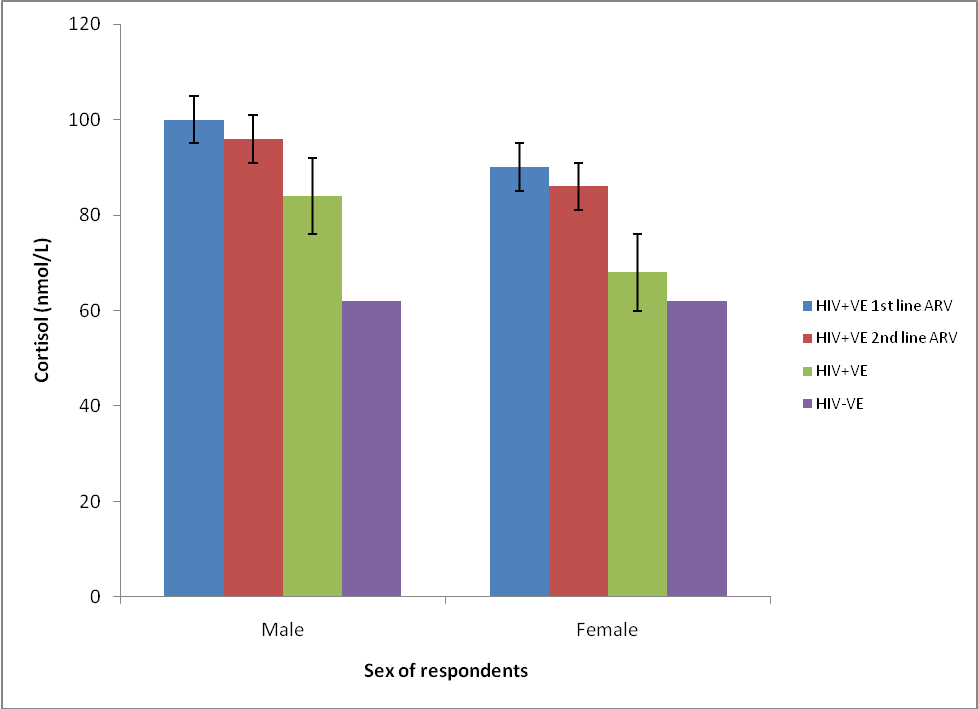 | Figure 1. Mean cortisol concentrations of HIV positive  and and  line ARV, HIV positive & negative male and female subjects line ARV, HIV positive & negative male and female subjects |
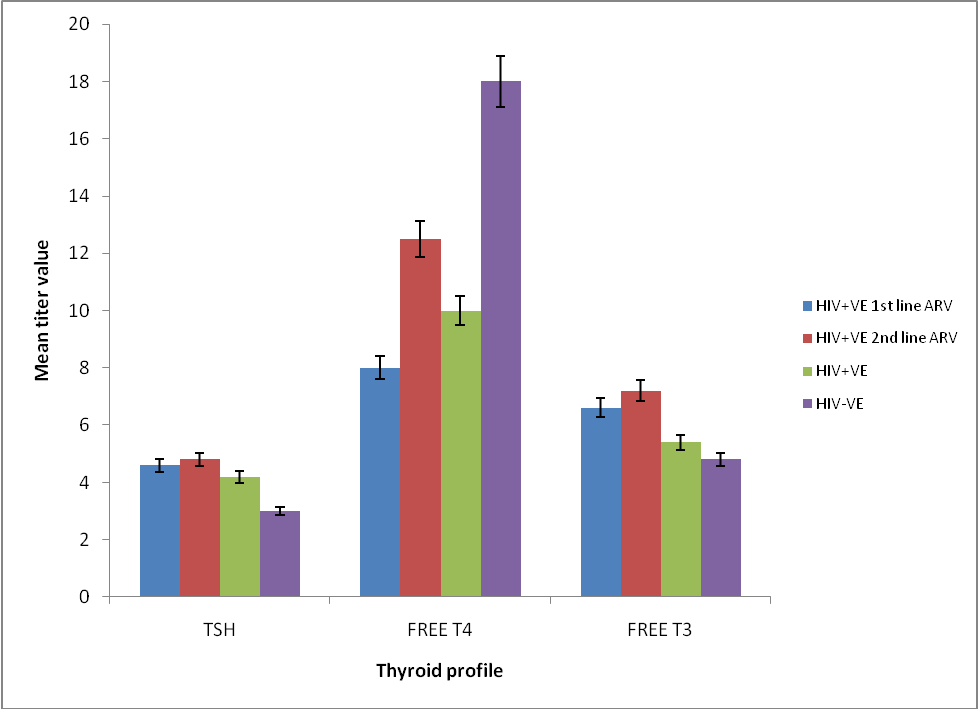 | Figure 2. Mean titer values of thyroid hormone profiles of HIV positive  and and  line ARV , HIV positive & negative male and female subjects line ARV , HIV positive & negative male and female subjects |
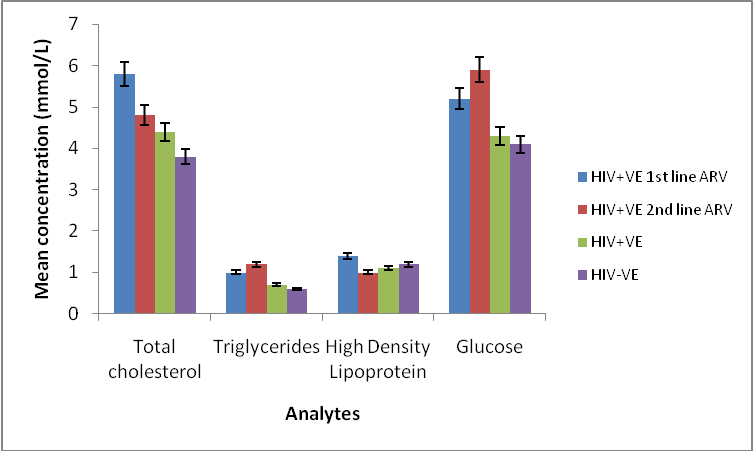 | Figure 3. Mean concentration of serum lipids and glucose profiles of HIV positive  and and  line ARV, HIV positive & negative male and female subjects line ARV, HIV positive & negative male and female subjects |
- Severe endocrine problems are often less seen since the advent of HAART, but subtle endocrine disorders are common with persons living with HIV on HAART. It can be challenging to diagnose endocrine problems in people with HIV because certain symptoms may be associated with altered levels of more than one hormone. For example fatigue and depression may be due low levels of thyroid hormone, cortisol, growth hormone, or testosterone [13, 30]. In addition, multiple endocrine mechanisms may interact in a complex syndrome such as wasting, lipodystrophy and other metabolic abnormalities [17].Patient on HAART regimen containing efavirence will benefit from periodic monitoring of cortisol as hypercortisolemia is commonly observed in stable HIV infection in the absence of clinical manifestations [31]. The prevalence of subclinical hypothyroidism is increased among HIV-positive patients on HAART, and this may warrant screening, especially for patients treated with stavudine [d4T] and patients with decreased CD4 cell counts [30]. HIV positive patients will benefit from having a complete hormonal study at baseline before commencing antiretroviral therapy and in future study, the drug combination should be taken into consideration so as to isolate the individual drug causing the hormonal imbalance [14, 16].
4. Conclusions
- Data of the present study show that both antiretroviral therapy and metabolic disturbances were associated with higher serum cortisol level of both male and female Nigerian subjects. Such subtle imbalances may have a major impact on the quality of life and many people with HIV may benefit from testing of hormone level and supplementation if appropriate. Although alterations in the metabolic pathways of cortisol by these drugs could account for our findings, the intimate mechanism responsible for these interactions remains to be elucidated.
ACKNOWLEDGEMENTS
- The authors are grateful to the management of the AIDS Prevention Initiative in Nigeria (APIN) clinic of the Lagos University Teaching Hospital (LUTH), Idi-araba, Surulere, Lagos, Nigeria, for the use of their research facilities and to all the participants, especially the HIV subjects, used in the study. We also appreciate Mr. Adeleke Atunnise for the statistical analysis of data obtained from the study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML