-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2014; 3(1): 9-18
doi:10.5923/j.ijvmb.20140301.02
Production Large Array of Cytokines by Mouse Dendritic Cells Pulsed with Hepatitis C Virus Pseudo-particles
Mohamed M. S. Farag
Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Nasr City, Cairo, Egypt
Correspondence to: Mohamed M. S. Farag, Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Nasr City, Cairo, Egypt.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Hepatitis C virus (HCV) E1 and E2 envelope glycoproteins (GPs) displayed on retroviral cores (HCVpp) are a powerful and highly versatile model system to investigate wild-type HCV entry and to study some steps of its life cycle. Dendritic cells (DC), the most potent antigen presenting cells (APC) found at trace levels in lymphoid and nonlymphoid tissues, are required for the priming of naive T lymphocytes. In the present study, we have analyzed different pattern of cytokines secreted by bone-marrow derived dendritic cells from BALB/c mice pulsed with pseudo particles from the hepatitis C virus. As earlier shown, in vitro pulsation with HCVpp successfully activated bone-marrow derived DC and, up regulation of MHCII, CD 86 and CCR-7 was demonstrated by FACS analysis. We now demonstrate that in vitro pulsation of DC with HCVpp elicited significantly higher secretion of DC cytokines including: IL-1α, IL-1β, IL-10, IL-12, IL-15, interferon-α (INF-α), interferon-γ (INF-γ), and tumor necrosis factor-α (TNF-α), but not IL-2, and IL-6. The presented data showed a significant highest production of cytokines, that plays a role in the impairment of DC function after HCV infection.
Keywords: Hepatitis C virus pseudo particles, Dendritic cell, Production of cytokines
Cite this paper: Mohamed M. S. Farag, Production Large Array of Cytokines by Mouse Dendritic Cells Pulsed with Hepatitis C Virus Pseudo-particles, International Journal of Virology and Molecular Biology, Vol. 3 No. 1, 2014, pp. 9-18. doi: 10.5923/j.ijvmb.20140301.02.
Article Outline
1. Introduction
- Worldwide, several hundred million people are infected with the hepatitis C virus [1]. It is a small, enveloped, positive-strand RNA virus that has been classified within the Hepacivirus genus, as part of the Flaviviridae family, which also comprises the genera Flavivirus and Pestivirus [2]. Progression to chronic disease occurs in the majority of HCV-infected persons. Infection is associated with an increased risk for liver disease and hepatocellular carcinoma and has become the main indication for liver transplantation. HCV infection also increases the number of complications in HIV infected people [3]. No vaccine is currently available to prevent new infections and the only treatment for chronic hepatitis C is interferon-α therapy, either alone or in combination with the guanosine analogue ribavirin. However, only ~40% of patients respond to treatment. Clearly, novel therapeutic strategies are urgently required as the health costs for HCV-infected people are predicted to spiral dramatically in the next few decades. Unfortunately, no efficient and reliable culture system is available to amplify the virus [4], preventing the elaboration of reliable infection assays. Recently, a model for HCV replication, based on the self-replication of engineered minigenomes in cell cultures, has been established [5, 6]. Although very useful in the study of HCV genomic replication, this system does not support production of HCV particles [7]. HCV virus-like particles have been generated in insect cells or, alternatively, by pseudotyping vesiculovirus or influenza virus particles with modified HCV E1 and E2 glycoproteins, harboring alterations in their transmembrane domains [8-11]. However, they were only poorly infectious or not at all, and inconsistencies in the results prevented their use in functional investigations of HCV cell entry [8, 9]. Therefore, new approaches are sorely needed to study HCV assembly and infection to design HCV cell entry inhibitors and to study the humoral immune response against HCV. Here, we sought to overcome these hurdles by developing infectious, genetically tagged HCV pseudo-particles (HCVpp) harboring unmodified E1 and E2 glycoproteins. High infectivity of these particles allowed the precise investigation of HCV E1 and E2 glycoproteins and their potential receptors in cell entry, HCV host-range, and neutralization by antibodies from HCV patient sera.Dendritic cells (DCs) are professional antigen-presenting cells within the immune system. They are continuously produced from hematopoietic stem cells in the bone marrow and are widely distributed, as immature DCs, into both lymphoid and nonlymphoid tissues [12-15]. Immature DCs, including epidermal Langerhan’s cells, splenic marginal zone DCs and interstitial DCs within nonlymphoid tissues, continuously sample self-antigen to maintain T cell self-tolerance [12]. Immature DCs can also take up foreign antigens. When triggered by pathogens, the pattern- recognition receptors expressed by immature DCs cause them to mature to immunogenic DCs. These mature DCs can initiate primary T cell–mediated immune responses because they express high amounts of cell surface major histocompatibility complexes (MHC) and costimulatory molecules [12-15]. Some studies suggest that DCs have the capacity to induce different types of T cell–mediated immune responses, depending on their lineage, maturation stage and activation signals. Dendritic cells are a major source of many cytokines, namely, interferon-alpha (IFN-α), interferon-γ (INF-γ), tumor necrosis factor-α (TNF-α), IL-1, IL-4, IL-6, IL-7, IL-10, IL-12 and IL-15 [16, 17]. They further produce macrophage inflammatory protein (MIP1g). All these stimulatory molecules are important in the elicitation of a primary immune response. Interleukin-12 production is critical for the promotion of an effective cellular immune response by activating and differentiating T lymphocyte to the Th 1 pathway [18]. These cytokines are critical for the differentiation and commitment of immune cells. For example, a Th1 cytokine IFN-γ in coordination with IL-12 induces Th1 cell differentiation [19, 20, 21]. Although these positive feedback mechanisms are essential for the profound differentiation of Th cells, the immune system also has a number of intrinsic and extrinsic machinery to antagonize the excessive differentiation of immune cells [22, 23]. In this study HCVpp were produced to stimulate bone-marrow derived DC from BALB/c mice in vitro, and this elicited significantly higher secretion of DC cytokines. This interaction between HCVpp and DC play an important role in the impairment of DC function after HCV infection, making them an interesting tool to produce immune response and protection for HCV infection.
2. Materials and Methods
2.1. Cells and Culture Conditions
- Huh-7 human hepatocellular carcinoma cell line and HEK293T cells were cultivated in DMEM cell culture medium (Invitrogen, Germany), supplemented with 10% (v/v) fetal calf serum (FCS) (Invitrogen, Germany), 2 mM L-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml (Invitrogen, Germany). Bone marrow derived dendritic cells were maintained in RPMI 1640 medium (Invitrogen, Germany) supplemented with 10 % FCS, 2 mmol/L L-glutamine, 100 U/mL Penicillin, 100 mg/mL streptomycin and 50 μmol/L 2-mercaptoethanol (Invitrogen, Germany).
2.2. Production of HCV Pseudotype Particles
- To generate HCV pseudotype particles (HCVpp), HEK293T cells were transfected with expression vectors encoding the viral E1/E2 glycoproteins [phCMVDCE1- E2(Con1)], human immunodeficiency virus (HIV)-Gag-Pol (pCMVDR8·74), and the packaging-competent green fluorescent protein (GFP)-containing retroviral transfer vector pHRCMV-Emd [24, 25]. For an expression control, we used the plasmid pcz vesicular stomatitis virus (VSV)-G instead of phCMVDCE1-E2(Con1). For transfection a calcium phosphate transfection kit was used (Clontech, Germany) [26], according to the manufacturer’s instructions, using 8 μg of each plasmid (ratio of 1:1:1) with 62 μl 2 M CaCl2 and added sterile water to get 500 μl. Then we added the calcium-DNA mix drop wise to 500 μl of phosphate buffer. This was carried out under continuously vortexing the phosphate buffer. After an incubation period of 20 minutes, we added again drop wise the DNA crystals to plated cells with confluency of 70–80%. Medium change was done 16 hours later. Supernatants containing HCVpp were harvested 40–48 h after transfection, clarified by lows peed centrifugation for 15 min, filtered through membranes with 0·45 mm pores and concentrated using Amicon Ultra-15 molecular filters with an exclusion size of 30 kDa (Millipore, Bedford, MA, USA).We usually concentrated the particles 20-fold and stored them at -80°C.
2.3. HCVpp Infection Assay
- Huh-7 human hepatocellular carcinoma cells were seeded the day before infection at 1 x 105 Huh-7 cells per well in a 12-well tissue culture plate (Greiner Labortechnik). One hour before infection, cells were washed three times with warm phosphate-buffered saline (PBS) and incubated with plain Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Germany). Then viral supernatants containing the HCVpp were added to the cells and incubated for 3 h. The supernatants were removed and the cells incubated in DMEM supplemented with 10% (v/v) fetal calf serum (FCS) (Biochrom, Germany) and 2 mm l-glutamine (Invitrogen, Germany) for 72 h at 37°C. The infectious titres, expressed as transducing units (TU)/ml, were determined as the percentage of GFP positive cells measured by fluorescence activated cell sorter (FACS) analysis using the formula analysis [(number of target Huh-7/volume of HCVpp)* (percentage of GFP-positive cells/100)]. Infected Huh-7 cells were trypsinized, suspended in PBS with 0·5% bovine serum albumin (Sigma-Aldrich, Germany) and analysed for GFP fluorescence by cytofluorometry.
2.4. Generation of Bone-marrow Derived Dendritic Cells (BMdDC)
- For preparation of bone-marrow, we used 6–10 week old BALB/c mice (Sulzfeld, Germany). They were maintained under SPF conditions and handled according to international guidelines. After sacrificing the animals, the tibia and femur bones were used to prepare bone marrow cells. With minor adaptations, cultivation of bone-marrow cells was done following the Inaba protocol [27]. On day 7 we pooled non-adherent and loosely adherent cells. The isolated cell suspensions were either taken for FACS analysis or plated into 12 well culture plates (Greiner Labortechnik) at a density of 1.5×106 cells per well in one ml of complete BMdDC medium (Invitrogen, UK).
2.5. FACS-Analysis of BMdDC
- Flow cytometry analysis for measuring the expression of different surface molecules was performed with a FACSCalibur® cytometer and data was analyzed with Cell Quest Pro software (Beckton Dickinson). For staining, 2×105 cells were incubated in staining-buffer (PBS and 0.5% BSA, Invitrogen) with either 1 µl of specific antibodies or the corresponding isotype control (APC anti-mouse CCR-7 (Biozol, Eching), R-PE anti-mouse CD 11c, FITC anti-mouse CD 86, FITC anti-mouse MHC II (I-Ad) (all from BD Bioscience, Germany) for 30 min on ice in the dark. Stained cells were pelleted for 3 min at 2000 rpm (Biofuge pico; Kendro, Hanau) and were washed twice with staining-buffer. A negative control with unstained cells was run first to determine the baseline fluorescence. Checking for unspecific binding, marker-setting was done with isotype controls. For instrument settings and compensation of R-PE and FITC, samples stained with individual fluorescent probes were used.
2.6. Activating BMdDC with HCVpp
- On day 7, FACS analyses revealed 77.3 to 81.3% of mature DC, which were placed in a 12-well culture plate with a concentration of 1.5×106 cells per ml. Twenty-four hours later, the DC were activated by adding nothing (negative control), HCVpp, 1 μg/ml E. coli lipopolysaccharide (LPS) (Sigma, St. Louis, MO), or HCVpp plus LPS together into the culture medium. Cells were harvested on day 9 and were washed extensively. Activation of DC was measured by FACS analyses.
2.7. Cytokine Production by Activated DC Cells
- Cell-free culture supernatants were harvested for DC alone, DC pulsed with LPS, DC pulsed with HCVpp, or DC pulsed with HCVpp and LPS for 24 hours for cytokines production. Appropriate dilutions assayed for IL-1α, IL-1β, IL-2, IL-6, IL-10, IL-12, IL-15, INF-α, INF-γ, and TNF-α. The detection was done by using mouse IL-1α, IL-1β, IL-2, IL-6, IL-10, IL-12, IL-15, INF-α, INF-γ, and TNF-α enzyme linked immunosorbent assay (ELISA) Ready-SET-Go kits (eBioscience, Germany). The actual cytokine concentrations in pg/ml were determined by using standard reagents as provided by the manufacturer.
3. Results
3.1. Production and Infectivity of HCVpp
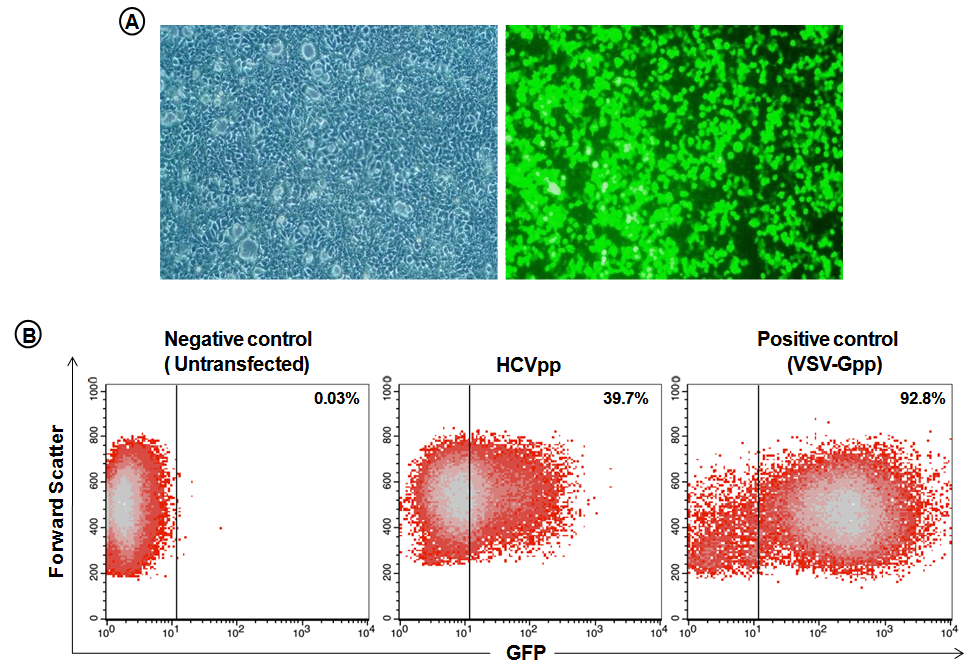
- HEK293T cells were transfected with expression vectors encoding the viral E1 and E2 glycoproteins, HIV retroviral core proteins and packaging-competent GFP-containing retroviral transfer vectors. HEK293T cells showed ~60-70% GFP positive cells 48 h after transfection indicating HCVpp production (Fig. 1A). Huh-7 human hepatocellular carcinoma cells were infected with HEK293T supernatants containing HCVpp or with VSV-G pseudotype particles as positive control, or left untransfected for negative control. Flow cytometry analysis showed 39.7% GFP-positive cells after infection with HCVpp-containing supernatant (Fig. 1B), demonstrating that the HCVpp preparation was indeed infective.
3.2. Flow Cytometry Analysis of Bone-marrow Derived Dendritic Cells (BMdDC)
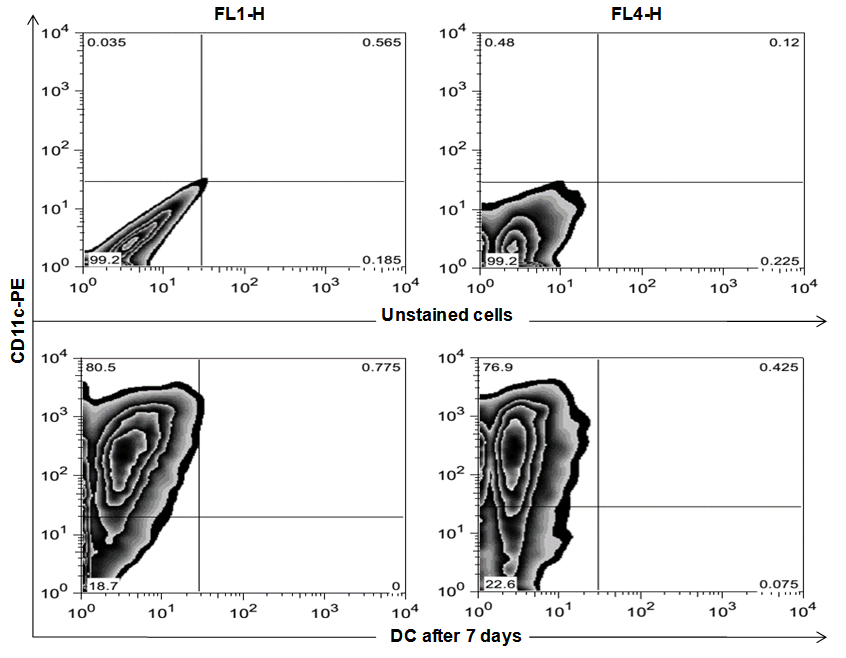
- To identify the differentiation of bone marrow towards the dendritic cell line, flow cytometry analysis of cultivated BMdDC was performed. Phenotype analysis of DC, identified by expression of CD11c showed 77.3% to 81.3% of the cultured cells were determined to be mature DC after 7 days of maturation with Granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-4 (Fig. 2).
3.3. Dendritic Cells are Strongly Activated by HCVpp in vitro
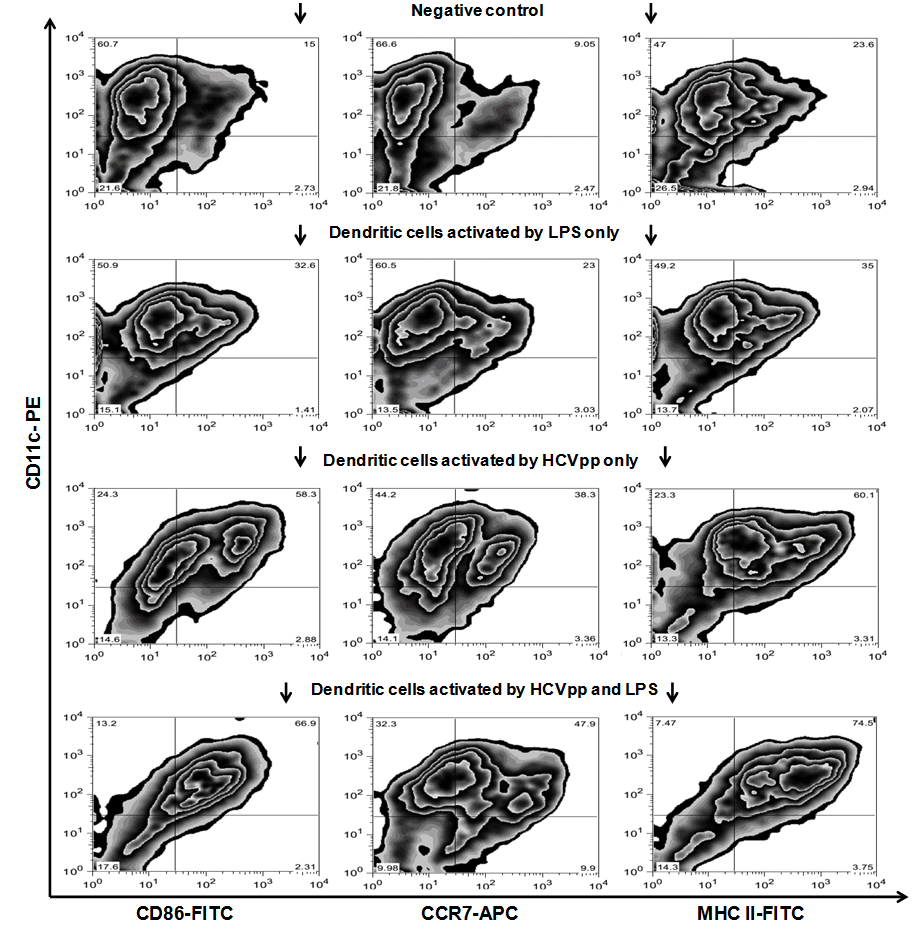
- As shown in Fig. 3 the DC in our study showed strong activation by HCVpp in vitro. Non-activated DC, specified by CD11c staining, presented CD 86 in 15%, CCR7 in 9.05%, and MHC II in 23.6%, in the FACS analysis. After 24 h of incubation with HCVpp and the co-stimulatory factor LPS these rates were increased to 66.9% for CD 86, 47.9% for CCR7, and 74.5% for MHC II, respectively. Incubation with LPS only leads to a middle alteration of the measured CD markers. Here CD 86 was found in 32.6%, CCR7 in 23% and MHC II in 35%, respectively. Incubation with HCVpp alone also resulted in an up-regulation of these markers, but lesser than found in the co-stimulated DC group. Here CD 86 was found in 58.3%, CCR7 in 38.3% and MHC II in 60.1%, respectively.
3.4. Enhanced Cytokines Secretion of DC after in vitro Exposure to HCVpp
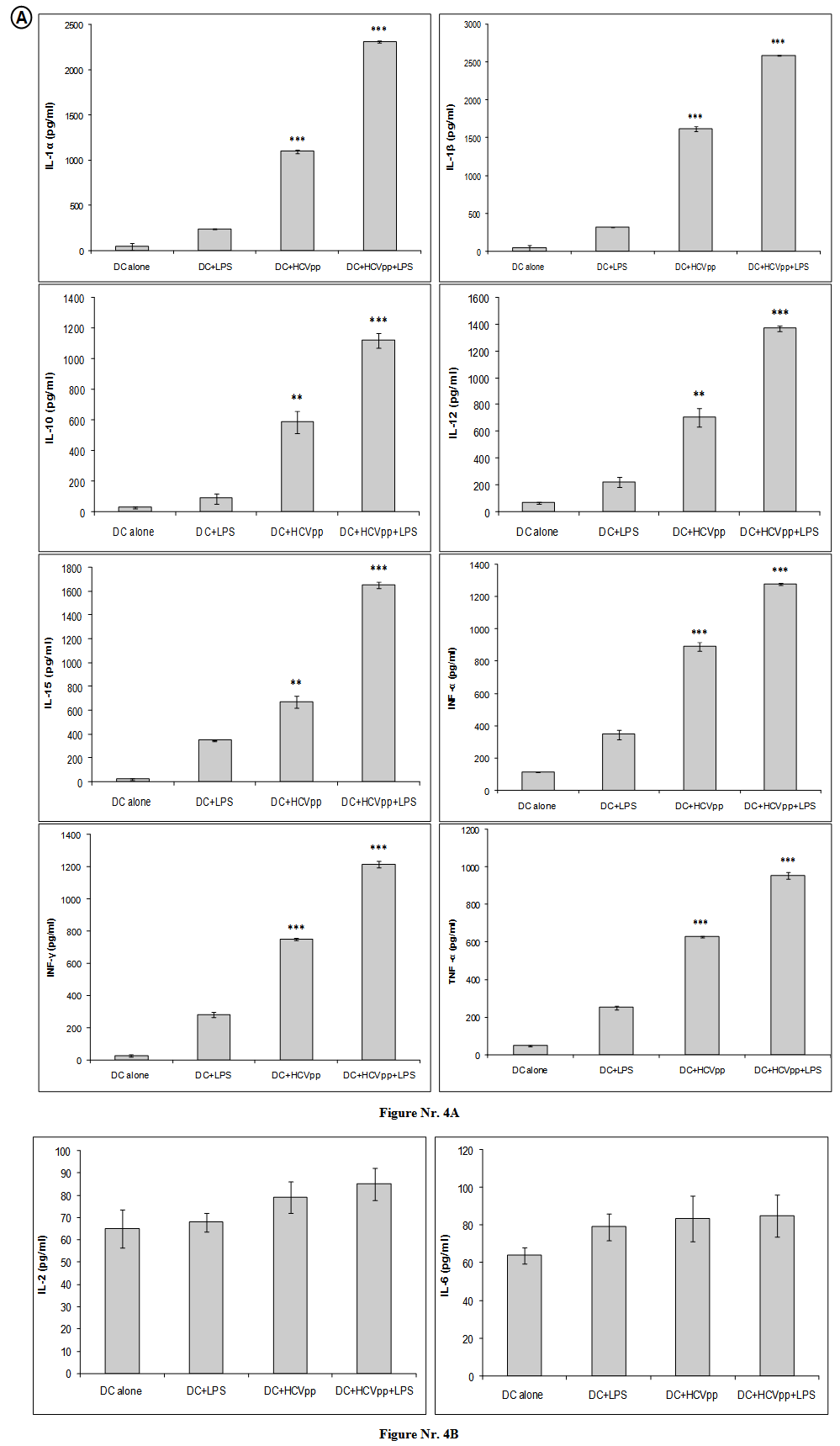
- Because the cytofluorometric analyses indicated an enhanced activation of DC cells by HCVpp or HCVpp and LPS, we studied functional aspects of DC cell activation. In culture supernatants of DC, we detected significantly larger amounts of the effector cytokines production including: IL-1α, IL-1β, IL-2, IL-6, IL-10, IL-12, IL-15, INF-α, INF-γ, and TNF-α. Our results showed that in vitro pulsation of DC with HCVpp or HCVpp and LPS elicited significantly higher secretion of DC cytokines. We have found three to fifth fold increases in signal intensity of the ELISA in activated DC groups comparing to negative control group. Our results showed that DC activated by HCVpp and LPS produced significantly (p < 0.001) highest amount for the following cytokines: IL-1α, IL-1β, IL-10, IL-12, IL-15, INF-α, INF-γ, and TNF-α (Fig. 4A). DC, previously incubated with HCVpp alone, were found to produce significantly (p < 0.001) highest amount of cytokines including: IL-1α, IL-1β, INF-α, INF-γ, and TNF-α (Fig. 4A). Regarding to IL-10, IL-12, and IL-15, DC activated with HCVpp alone showed the second highest secretion for theses cytokines (p < 0.05) (Fig. 4A). Unlike many other previous cytokines, IL-2, and IL-6 did not produced by activated DC groups (Fig. 4B).
4. Discussion
- We have studied herein different patterns of cytokine produced by DC generated in vitro and pulsed with HCVpp or HCVpp and LPS for 24 hours. The analysis was concentrated initially on the activation of BMdDC with HCVpp and production of a large array of cytokines in vitro. As earlier shown, in vitro pulsation with HCVpp successfully activated bone-marrow derived DC, up regulation of MHCII, CD 86 and CCR-7 were demonstrated by FACS analysis [28]. We now demonstrate that in vitro pulsation of DC with HCVpp elicited significantly higher secretion of different DC cytokines. The present study demonstrated that 1) DCs are strongly activated by HCVpp in vitro, and produce a large array of cytokines known to contribute to T cell priming (IL1-α, IL1-β, IL-15, TNF-α) or to T cell maturation (IL-12, INF-α, INF-γ); 2) different signals lead to a different regulation of the production of immunologically relevant cytokines such as IL-12; 3) finally, production of IL-10; a key immunomodulator. Among the various cytokines tested, DC pulsed with HCVpp produced IL1-α, IL1-β, IL-15, and TNF-α cytokines that potentially regulate naive T cell activation. This is in agreement with the reported production of IL-1a and IL-1b within Langerhans cells and peripheral blood DC [29, 30-32]. The secretion of IL-1b by DC seems to be essential for induction of primary immune responses in skin [33]. Activated DCs were found to produce TNF-α which may regulate the capacity of DC to initiate an immune response. IL-15, a recently identified cytokine with IL-2-like properties, was shown to increase Ag-specific T cell activity [34]. The production of IL-15 by DC pulsed with HCVpp is in line with description of this cytokine in Langerhans cells and in human blood-derived DC [35]. Furthermore, human blood DC were reported to produce functional IL-15 protein with chemotactic activity for T cells [36]. IL-12 is a heterodimeric molecule produced by antigen presenting cells (APCs) that appears to be central in promoting Th1 differentiation through induction of IFN-g production [37]. It is not yet clear whether IL-12 p35 and p40 chains are expressed constitutively in APCs, but both chains need to be assembled to form biologically active IL-12 protein [38]. Our results are in agreement with a previous study reporting constitutive IL-12 p40 and p35 secretion in DC, and secretion of an active IL-12 p70 [39]. DCs have a crucial role in determining the type of T cell mediated response [40, 41]. IL-12 is an important immune modulatory molecule, which specifically promotes Th1 cell differentiation and suppresses Th2 cell function, and induces a Th1 cell immune response [42]. In this study, HCVpp could promote DCs to secrete IL-12p70 in vitro. This indicated that HCVpp might enhance Th1 cell immune responses by promoting DC to secrete IL-12. And then Th1 cells can induce the proliferation of CTLs and amplification of CD8+ T cell responses [43]. Furthermore, activated DCs were found to produce IL-10 suggests a particular role of this DC subset in the priming of naive T cells. Thus, IL-10 might be involved in controlling the levels of T cell activation induced by DC [44]. Furthermore, IL-10 has been shown recently to directly act on T cells to induce a state of anergy [45]. Moreover, by analogy to IL-10 effects on monocytes [46], endogenous IL-10 may down-regulate the production of IL-1a, IL-1b, TNF-a, and GM-CSF by DC. Our data showed that IL-2, and IL-6 were found unexpectedly to be unexpressed by DC pulsed with HCVpp. Our results are in agreement with a previous study reporting down regulation of IL-2 expression and production by human DC [47].We and others have demonstrated that it is possible to specifically activate DC in vitro against HCVpp and, up regulation of MHCII, CD 86 and CCR-7 was demonstrated by FACS analysis [28]. In this study, we showed that the interaction between DC and HCVpp in vitro produced significantly highest amount of cytokines that are immunologically relevant. The function of DC differs according to their localization and level of maturation. In general these cells are strong stimulators of the allogenic mixed leukocytes reaction, induce antigen specific humoral and cellular immune responses, produce and induce a variety of cytokines of both Th1 and Th2 types, ensure the survival and activity of the antigen-specific lymphocytes including CTL and induce immunogenic tolerance [48, 49]. HCVpp were chosen to activate the DC because they contain the E1 and E2 proteins and present them as closely to mature virions as possible. Due to that, neutralizing epitopes of the E1 and E2 proteins are potentially presented in the natural three-dimensional fashion. DC were chosen since we and others showed that they can be used to strongly induce immune responses which exceed the responses achieved by immunization with proteins or peptides only [50]. LPS was chosen because it has been shown potential in vaccinating, easy storage and wide application, with both carrier and adjuvant functions that activate DC [51]. It is well known, that LPS is used as co-stimulatory factor for DC [52]. In conclusion, in vitro pulsation with HCVpp successfully activated bone-marrow derived DC, up regulation of MHCII, CD 86 and CCR-7 were demonstrated by FACS analysis. Activated DC secretes a large array of soluble factors, including several cytokines that are immunologically relevant. However, the effect was much higher when HCVpp pulsed DC was done in presence of the co-stimulant LPS, which was needed to significantly increase DC activating surface markers and cytokines production. It is therefore likely that the outcome of a primary immune response will be affected not only by the subset of DC involved, but also by the activation signal engaged during the initiation phase of the response. Production of a large array of DC cytokines could be playing a role in the impairment of DC function after HCV infection. We believe that use of DC as a cellular based therapy is of great interest and should be evaluated further to sufficiently fight chronic HCV infection. DC function in HCV patients could be restored in vitro, possibly assisting the patients to better control or even clear the virus.
ACKNOWLEDGEMENTS
- We thank Professor Dr. Rolf Bartenschlager, University of Heidelberg and his group for their kind gift of HCV expression plasmids.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML