-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2014; 3(1): 1-8
doi:10.5923/j.ijvmb.20140301.01
Infectobesity in the Polish Population - Evaluation of an Association between Adenoviruses Type 5, 31, 36 and Human Obesity
Iwona Bil-Lula1, Sylwia Stąpor2, Marta Sochocka3, Maria Wołyniec4, Katarzyna Zatońska4, Rafał Ilow5, Andrzej Szuba6, Grzegorz Sawicki7, Mieczysław Woźniak1, 7
1Department of Clinical Chemistry, Wroclaw Medical University, Wroclaw, 50-556 Wroclaw, Poland
2Laboratory of Haematological and Transplant Diagnostics, University Hospital No 1, Wroclaw, 50-369, Poland
3Ludwik Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Science, Wroclaw, 53-114, Poland
4Department of Social Medicine, Wroclaw Medical University, Wroclaw, 50-345, Poland
5Department of Food Science and Dietetics, Wroclaw Medical University, Wroclaw, 50-556, Poland
6Department and Clinic of Internal and Occupational Diseases and Hypertension, Wroclaw Medical University, 50-556, Poland
7Department of Pharmacology, University of Saskatchewan, Saskatoon, SK S7N 5E5, Canada
Correspondence to: Iwona Bil-Lula, Department of Clinical Chemistry, Wroclaw Medical University, Wroclaw, 50-556 Wroclaw, Poland.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The main aim of this study was to determine an association between serum antibodies against adenoviruses (AdVs) type 5, 31 and 36 (as positive control) and obesity in the Polish adults and the evaluation of an association between anti-AdV antibodies status and body mass index (BMI), anthropometric measures, serum lipids and C-reactive protein (CRP). The study included 200 adults, both obese and non-obese. Serum neutralization test was used to assess the presence of anti-AdV antibodies and routine serum chemistry, leptin, CRP was evaluated. The prevalence of anti-AdV5, 31, 36 antibodies ranged from 6.8% to 31.3%. We demonstrated an association between immune response to AdV infection (by establishment of anti-AdV31 and anti-AdV36 antibodies in serum) and obesity in adult Poles. Higher BMIs and WHR (waist to hip ratio) or waist circumference were found in infected versus uninfected p<0.05. All AdV-positive subjects were older than uninfected control, but sex had no influence on the prevalence of antibodies. We noticed that the presence of anti-AdV31 and 36 antibodies was associated with changes in lipid metabolism but kind and severity of lipid metabolism disturbances differed due to the type of infecting virus. Neither elevated CRP nor decreased leptin levels were related to infectobesity. Infections of AdV31 and AdV36 may be associated with obesity in the Polish population and AdV5 is not linked with human obesity.
Keywords: Adenoviruses, Obesity, Antibodies
Cite this paper: Iwona Bil-Lula, Sylwia Stąpor, Marta Sochocka, Maria Wołyniec, Katarzyna Zatońska, Rafał Ilow, Andrzej Szuba, Grzegorz Sawicki, Mieczysław Woźniak, Infectobesity in the Polish Population - Evaluation of an Association between Adenoviruses Type 5, 31, 36 and Human Obesity, International Journal of Virology and Molecular Biology, Vol. 3 No. 1, 2014, pp. 1-8. doi: 10.5923/j.ijvmb.20140301.01.
Article Outline
1. Introduction
- Overweight and obesity are one of the most serious public health challenges of the 21st century for the World Health Organization. Obesity is recognized as a chronic disease leading to higher risk for cardiovascular disease, diabetes, orthopedic problems and mental disorders[1-8]. It is well known that obesity has multifactor etiology but although all these are well recognized, they cannot explain the rapid change in prevalence of obesity in the world[1, 3, 4, 8, 9]. Alongside the traditionally recognized causes of obesity, since year 1982 attention of scientists has been focused on viral infections as possible cause of obesity[10-18]. In year 1992, the first mention of the role of adenoviruses in the development of obesity appeared[14]. Studies involving animals confirmed a strong association between adenovirus 36 (AdV36) and increased predisposition to excessive accumulation of visceral fat[14, 19-22]. However, although experimental infections in animals or model cell lines showed an adipogenic potential of some AdV types, there are only few controversial reports on the role of these viruses in the development of obesity in humans and the knowledge about engagement of other AdV types is still limited[14, 19-21, 23-28]. The study including 502 participants from the USA showed a significantly greater prevalence of AdV36 in obese people (30%) than in non-obese (11%). The BMI of AdV36-seropositive obese and non-obese participants was significantly higher but serum cholesterol and triglyceride concentrations were much lower than of their respective seronegative counterparts[23]. A study that screened human twins for the presence of AdV36 antibodies also revealed that the AdV36-seropositive twin had a higher BMI and more body fat than his or her seronegative sibling[23]. In this study we focused on adenoviruses because their worldwide distribution. An incentive to undertake this study was also distinct report published by Goossens et al (2011)[26] and Broderick et al. (2010)[29], who demonstrated lack of evidence for the role of AdV36 in obesity in a European cohort and USA military recruits. These findings prompt us to check the hypothesis that adenoviral infections might be associated with obesity in specific populations. Most adenovirus infections are acquired in a childhood, but adenovirus DNA can be detectable in lymphocytes of adults, which suggests the possibility of the recurrence or the persistence of latent infections after many years since primary infection. Therefore the purpose of this investigation was to determine an association between presence of specific anti-adenovirus antibodies against AdV5, 31 and 36 (as positive control) and obesity in the Polish population. We decided to test seropositivity for these viruses to indicate evidence of contact with the virus and adequate reaction of immune system. We chose AdV31 (group A) due to little information about an association of AdV31 with obesity available in the literature[30, 31]. Adenoviruses from group C are phylogenetically the oldest; therefore they are the most different from other AdV groups [30]. Because there are some reports excluding AdV2 (C) from the group of potential etiological factors of obesity of infectious origin[23], as pioneers, we evaluated if the infection of AdV5 (C) is associated with development of human obesity[32]. Therefore, the main aim of this study was to determine an association between adenoviruses type 5, 31 and 36 and obesity in the Polish population.
2. Materials and Methods
- Two hundred adults, both obese (50%) and non-obese (50%) were included in the study. Obese subjects were recruited by the Department of Social Medicine, Wroclaw Medical University and non-obese volunteers were collected by Department of Clinical Chemistry, Wroclaw Medical University in Poland. Written informed consent was obtained for collection of blood from all study participants and the protocol was approved by local Ethics Boards (no.115/2011).
2.1. Classification of the Subjects
- Subjects with BMI≥30 kg/m2 were defined as obese. In accordance with recommendations of IDF (2006), women and men characterized by waist circumferences ≥80 cm (WHR>0.8) and ≥94 cm (WHR>1.0) respectively, were classified as subjects with central obesity[33].
2.2. Determination of Serum Antibody Status
- The presence of specific anti-AdV5, AdV31 and AdV36 neutralizing antibodies in serum was assessed using serum neutralization test (SN). Human adenoviruses type 31 (VR-1109), 36 (VR-1610) were purchased from ATCC (Manassas, Va, USA) and AdV5 was provided by Institute of Immunology and Experimental Therapy, Wroclaw. The viruses were grown on A549 cells in Dulbecco Modified Engels Medium supplemented with FBS and antibiotics, under stringent aseptic conditions, at 37°C, 5% CO2 for 3-4 days and TCID-50 (the dose of virus that produces CPE in 50% of wells) was determined for each virus. The viruses were grown on cell line repeatedly to obtain homogenous virus stock and to enhance the virus strength for further SN. The viruses were detected by means of PCR reaction, with primers complementary to AdVs DNA, as described previously[34]. Culture supernatants were frozen at -80°C to preserve the native properties of viruses.
2.3. Serum Neutralization Test
- The presence of specific anti-adenovirus antibodies in human serum was tested by checking whether serial diluted serum is able to protect A549 cells from cytopathic effect of added virus, as previously described[19]. Briefly, the serum dilutions ranged from 1:2 to 1:512. One hundred TCID-50 of the respective adenovirus work stock was added to each of the wells. Samples were assayed in duplicate, and controls for serum (serum and cells, no virus), cells (cells only), and virus (cells and virus, no serum) were included in each assay. Plates were incubated at 37°C for 7 days. The presence of CPE was noted mainly after 3-4 days (depending on the type of AdV). Serum samples showing no CPE in dilutions of 1:8 or higher, in both duplicates, after 7 days of incubation were considered positive for neutralizing antibodies to the respective virus. CPE in virus control wells, and the absence of CPE in serum control and cell control wells, was ascertained.
2.4. Measurement of Serum Lipids
- Serum samples were assayed for cholesterol, triglycerides, high-density lipoproteins (HDL) and low-density lipoproteins (LDL) using commercial tests from BioMaxima (Lublin, Poland).
2.5. Measurement of Leptin and CRP Concentrations
- Leptin and C-reactive protein (CRP) concentrations were assayed in plasma obtained from fasting blood samples collected in a morning. Concentrations of leptin/CRP were determined by using of Human Leptin ELISA and Human CRP Elisa Kits (RayBio, USA) according to the manufacturer’s instructions. Each sample was analyzed in duplicate.
2.6. Statistical Analysis
- Statistical analysis was performed using Statistica v.8.0 (StatSoft, Krakow, Poland). The Chi square test or Fisher’s exact test were used for a univariate analysis. Means of the variables were compared by Test T or Mann-Whitney test in infected versus uninfected subjects. We also determined if antibody status was associated with severity of obesity by means of correspondence analysis. The correlations between AdV serological status and serum lipids/leptin were assessed by Spearman's rank correlation test. P value of <.05 was considered to be significant.
3. Results
3.1. The Prevalence of Antibodies
- Two hundred adults were tested for anti-AdV5, 31 and 36 antibodies in the serum. AdV36 was treated as positive control (adipogenic virus) due to previous reports[22-24]. Subjects, whose serum samples were positive for antibodies to more than one type of adenovirus were excluded from groups in which results were positive but were included into group in which results were negative. Of 82 subjects tested for anti-AdV5 antibodies, 13 were positive for anti-AdV5 antibodies yielding a cumulative incidence of 15.9%. 26 (31.3%) of 83 adults tested for antibodies against AdV31 were positive and only 6 of 88 (6.8%) subjects were positive for anti-AdV36, respectively (Table 1). The prevalence of anti-AdV antibodies was higher in obese than non-obese subjects, except AdV5 infection (Figure 1). The median age of AdV seropositive adults was significantly higher, beyond AdV5, than the median age of subjects not infected: AdV31 (49 vs 25 years) and AdV36 (60 vs 25 years), p<0.05.
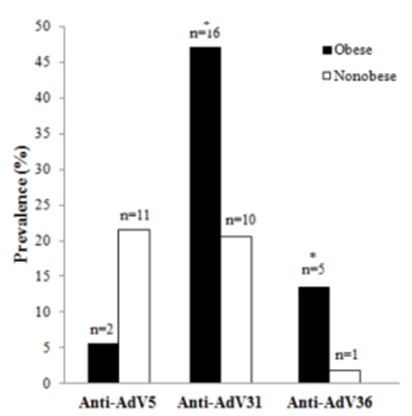 | Figure 1. The prevalence of anti-adenovirus antibodies in obese and non-obese subjects. AdV- adenovirus; * p<0.05 |
3.2. Anthropometric Indicators
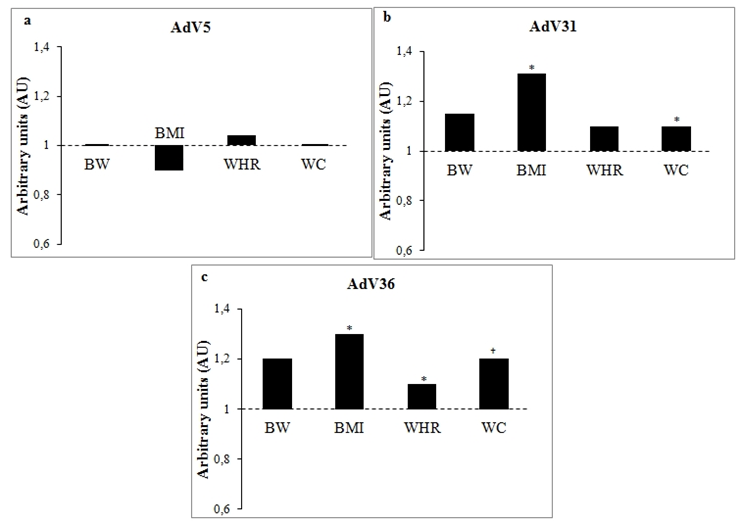
- Subjects who were AdV5, 31 and AdV36-positive did not have greater median weights in comparison to subjects AdV-negative, respectively: 75.0 vs 75.0 kg, 80.5 vs 70.0 kg and 87.8 vs 74.1 kg (Table 1). Nevertheless, the median BMI in adults infected with all tested viruses (beyond AdV5) was greater than was seronegativity (p<0.05) (Table 1, Figure 2).
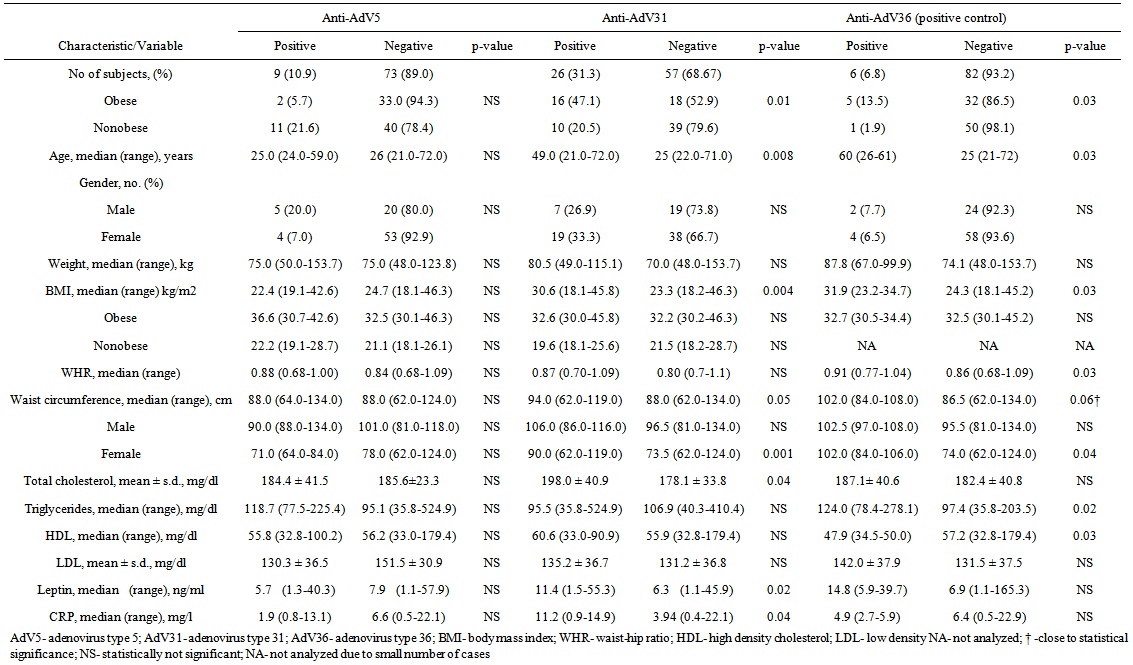 | Table 1. Characteristic of AdV5, 31, 36 Positive/Negative Subjects |
3.3. Anti-Adenovirus Antibodies vs Serum Lipid Profile and Leptin/CRP Levels
- We analysed if the presence of anti-adenovirus antibodies in serum is related to changes in serum lipids profile. The values of triglycerides in anti-AdV36 antibodies positive subjects were greater than in anti-AdV36 antibodies negative (124.0 vs 97.4 mg/dl, p=0.02) and interestingly they had also much lower level of serum HDL (47.9 vs 57.2 mg/dl, p<0.05). Anti-AdV31 antibodies positive humans presented only elevated level of total cholesterol in comparison to their respective seronegative counterparts (Table 1). In a case of AdV5 infection, we did not reveal any relation between seropositivity and changes in lipids concentrations (p>0.05). To determine whether the pathogenesis of infectobesity underlies reduction of leptin secretion in AdV-infected adipocytes, the serum concentration of leptin was assessed. The presence of anti-AdV31 specific antibodies was associated with elevated levels of the leptin (Table 1). As it was expected we noticed a strong positive correlation between serum leptin and BMI (r=0.50, p<0.05) or body weight (r=0.36, p<0.05) and much elevated levels in obese than non-obese: median 20.4 ng/ml vs 4.3 ng/ml, p=0.000, respectively. We also did not confirm an association between anti-AdV antibodies and leptin in serum of obese anti-AdV antibodies positive vs negative and nonobese anti-AdV antibodies positive vs negative. Because our data excluded the role of leptin in the development of infectobesity, we decided to estimate if the cause of AdV-dependent obesity underlies chronic inflammation due to AdV infection. For this reason we assessed the serum level of CRP as a first-line marker of inflammation. Interestingly, CRP level was higher in anti-AdV31 antibodies positive subject in comparison to their negative counterparts (Table 1). However, as it was expected, CRP was higher in obese than non-obese (11.0 vs 1.23 mg/l, p=0.000). In the further analysis, we used the Spearman's rank correlation test to evaluate correlations between AdV serum status and lipid/leptin profile. We noticed correlation between triglycerides and HDL (r=-0.59, p<0.05), LDL and leptin (r=0.34, p<0.05) in subjects anti-AdV31 antibodies positive. We did not observe these relations in negative control. In comparison of anti-AdV36 antibodies positive/negative groups we reported that decreased level of triglycerides was accompanied by decreased level of HDL (r=-0.64, p<0.05) in anti-AdV36 antibodies positive individuals inversely to anti-AdV36 antibodies negative subjects.
4. Discussion
- Because of ethical reason, the targeted viral infection in human is not possible. There are many reports suggesting significant association between adenoviruses and animal obesity[14, 19-21, 22], but studies indicating relation between adenoviruses and human obesity are still controversial. Moreover, most of worldwide reports focused on AdV36 and little was told about other AdV types[21, 23, 24, 28, 35]. Data from this study indicated that the prevalence of anti-AdV5, 31 and 36 antibodies ranged from 31.3% (AdV31) to 6.8% (AdV36) in the Polish study group. We demonstrated an association between presence of anti-AdV31 and 36 antibodies in the serum and obesity in those subjects. Infections of AdV36 and 31 were observed 5- and 1.6-fold, respectively, more likely in obese than in non-obese lending support to hypothesis of obesity due to adenovirus origin. Atkinson et al. (2005) reported no difference in prevalence of anti-AdV31 antibodies in obese vs non-obese[23]. It is interesting that prevalence of antibodies specific for AdV31, AdV36 was much higher in groups from North America (70% vs 80 and 30% vs 11%)[23] in comparison to subjects in the Polish population (47.1 % vs 20.5%, 13.5% vs 1.9%). Different prevalence of above infections in both compared studies indicates that although it is said that adenoviruses have worldwide distribution, the prevalence of adenoviral infections may vary by the geographic location. Moreover, Whigham et al. (2006) reported that only 71% of AdV37 inoculated chickens developed specific antibodies, hence it is possible that AdV37 has lower potential to cause infections[36]. Because BMIs of anti-AdV antibodies positive and anti-AdV antibodies negative obese even as non-obese participants were similar, these data prompted us to reflect on the possibility that the reported association of AdV with human obesity could result from a greater susceptibility to infection in obese individuals. On the other hand, the study of anti-AdV5 antibodies status argues against this hypothesis. We noticed that prevalence of anti-AdV5 antibodies was similar in obese and non-obese participants. Following this hypothesis, if obese subjects were infected with pathogens more often than lean people, they should be infected with AdV5 more often due to high distribution of AdV5 in the human population, respiratory and droplet transmission and long-time stability in the environment[14, 31, 32, 36-42]. The fact that at least AdV2 and AdV5 are not associated with human obesity suggests that the adipogenic associations of AdV31 and 36 are probably not a non-specific response to any infection. The evidence for association of above adenoviruses with increased weight and adiposity in humans is also provided by our simultaneous in vitro studies involving 3T3L1 cells. We noticed that cells inoculated with AdV31 and 36, not AdV5, demonstrated an altered differentiation and maturation and increased accumulation of fat (data not shown).We noticed that subjects positive for antibodies against AdV31 and 36 had significantly higher median BMIs than those of negative. Our results are contrary to those published by Atkinson et al. (2005) who showed no relationship between elevated BMI and AdV31 infection in human twins[23, 32]. Our further investigation revealed that AdV infections were associated with severity of obesity or overweight within the subgroup of obese and non-obese due to higher prevalence of anti-adenovirus antibodies in subjects with BMI≥35 kg/m2 and BMI≥25 kg/m2. As it was expected, WHRs in participants previously infected with AdV36 were greater than in those who did not possess anti-adenovirus antibodies in the serum. This observation was similar to results obtained by Atkinson et al. (2010) who examined obese children infected with AdV36[24]. Additionally AdV31 and AdV36-antibodies positive groups presented central obesity. Waist circumferences of subjects positive to anti-AdV antibodies were higher in comparison to their negative counterparts and indicated increased accumulation of fat in the abdomen. This association was evident in AdV-antibodies positive women (p<0.05) and less pronounced in anti-AdV antibodies positive males who usually have central obesity. Data showed that subjects seropositive to AdV31 and AdV36 were older than seronegative control, but sex had no influence on prevalence of antibodies. It is known that adenoviruses are able to cause a persistent asymptomatic infection and reactivation in different tissues after long period of latency[31, 37, 42]. For this reason they might be associated with the development of obesity as a chronic disease. Previous studies presented controversial reports with regard to changes in serum lipids due to AdV infection[14, 21, 23, 35, 44, 45]. It may suggest that there are some different mechanisms underlying lipid disturbances in different species, the adipogenic and lipidemic effects may by mediated via separate pathways and animal or in vitro models may not be appropriate to forecast lipid changes in humans. In this study we noticed that the presence of AdV31 and 36 infections was associated with changes of lipid metabolism but kind and severity of disturbances might differ due to type of infecting virus. The proof of that are differences in serum lipids in subjects previously infected with different types of adenoviruses. Other reports, including animal and 3T3L1 in vitro studies suggested that adenoviral infection leads to suppressed expression and secretion of leptin in adipose tissue, as the pathogenesis of “infectobesity”[22]. In our study, anti-AdV31 antibodies positive subjects had elevated level of serum leptin probably as a function of BMI and greater amount of fat tissue. Data showed no difference in concentration of serum leptin in obese anti-AdV antibodies positive vs obese negative and in non-obese antibodies positive vs negative confirming that elevated leptin levels are due to much greater fat deposits in obese, not due to AdV infection. It is known that serum leptin level reflects body lipid content and strongly correlate with adipocyte volume[11, 46-48]. Because hypoleptinemia was reported in studied involving animals and in vitro studies, it should be pointed out that most in vivo experiments demonstrated decreased leptin expression per unit of adipose tissue. Therefore, the experiment reported by Vangipuram et al. (2007) showed paradoxical elevated levels of circulating leptin accompanying reduced leptin expression[22]. We also did not confirm that the increased central obesity was the result of chronic low-grade inflammation due to adenoviral infection in the adipose tissue. We noticed that increased level of CRP was the function of BMI and amount of body fat, than AdV infection. Surprisingly we noticed decreased serum level of CRP in anti-AdV36 antibodies positive obese in comparison to anti-AdV36 antibodies negative obese. This confirms that the inflammation associated with obesity is not related with adenoviral infection and AdV36 may be associated with decreased secretion of CRP in adipocytes or liver, but this needs further investigations.So et al. (2005) provided a study on the role of AdV5 in the development of obesity in mice[49]. As pioneers we demonstrated that presence of anti-AdV5 antibodies in the serum was not associated with obesity in humans. These data confirmed our suggestions that AdV5 as previously reported AdV2 (from the same group) are not related to human obesity[26]. Our unpublished in vitro studies also documented that AdV5 inoculation of 3T3L1 did not produce increased accumulation of intracellular fat (data not shown). Moreover, incidence of adenovirus infections peaks in infants and children between the age of 6 months and 5 years and more than 80% of human population was affected with adenoviral infections. Therefore it is obvious that not all types of human adenoviruses are associated with the development of obesity, because then the most of human populations should be overweight or obese. Additionally, an opposite results of study reported by So et al. (2005) may suggest that mice are not appropriate animal model to predict effects of AdV infections in humans[49].
5. Conclusions
- In conclusion, we showed that there is an association between immune response to AdV31 and 36 infections (presence of anti-AdV antibodies in serum) and obesity in the Polish population. We are aware that conclusively determine a causative role of adenoviruses in human obesity is very difficult. Indirect linking an episode of a particular infection to obesity developed later in life is risky. Moreover, even if SN test is considered as “gold standard” for virus typing there is always possibility of cross-reactions with AdV-type of similar fenotype. Therefore the intention was to point out the possible association between immune response to adenoviral infection and the development of obesity but we are also aware that perhaps AdV infections increase adiposity only in the presence of other risk factors. While studies of anti-AdV seropositivity carry information about the possible association with obesity, they do not explain underlying mechanism of increased fat accumulation. Therefore, in our opinion, long-term observation of lean volunteers spontaneously infected with AdVs is needed. Data obtained from this study might give a perspective that immune response to infections with mentioned AdV types might promote adiposity in the Polish population. In our opinion, this concept alerts clinicians to the possibility of infectobesity and promotes further research, leading to the development of new prevention and treatment strategies.
ACKNOWLEDGMENTS
- This research was sponsored by the Polish Ministry of Science and Higher Education (Wroclaw Medical University grant no. Pbmn/44).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML