-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2013; 2(1): 7-15
doi:10.5923/j.ijvmb.20130201.02
Activation of Dendritic Cells by Hepatitis C Virus Pseudo-Particles Containing Functional E1–E2 Envelope Protein Complexes in Vitro
Mohamed M. S. Farag
Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Nasr City, Cairo, Egypt
Correspondence to: Mohamed M. S. Farag, Botany and Microbiology Department, Faculty of Science, Al-Azhar University, Nasr City, Cairo, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Dendritic cells (DCs) are a promising tool for therapeutic and/or prophylactic vaccination experiments, considering their multiple functions in immune modulation. We used bone-marrow derived dendritic cells from BALB/c mice pulsed with pseudo particles from the hepatitis C virus to activate dendritic cells in vitro. Hepatitis C virus pseudo particles (HCVpp) consist of the genotype 1b derived envelope proteins E1 and E2, covering a non-HCV core structure. Thus, not a single epitope, but the whole “viral surface” induces immunogenicity. HCV E1/E2 pseudo particles were successfully established, demonstrated by Huh-7 transduction assay. Our results indicate that mouse DC can efficiently be activated in vitro using HCVpp, identified by FACS analysis showing increased CD86, MHCII, and CCR-7. However, the effect was much higher when HCVpp pulsed DC was done in presence of the co-stimulance LPS, which was needed to significantly increase DC activating surface markers. These data lead to the assumption that DC function of HCV infected patients could be restored in vitro, possibly assisting the patients to better control or even clear the virus.
Keywords: Hepatitis C Virus Pseudo Particles, Dendritic Cell, Cell based Therapy
Cite this paper: Mohamed M. S. Farag, Activation of Dendritic Cells by Hepatitis C Virus Pseudo-Particles Containing Functional E1–E2 Envelope Protein Complexes in Vitro, International Journal of Virology and Molecular Biology, Vol. 2 No. 1, 2013, pp. 7-15. doi: 10.5923/j.ijvmb.20130201.02.
Article Outline
1. Introduction
- Little is known about the ability of hepatitis C virus (HCV) to alter early innate immune responses in infected patients. Despite many developments and improving results in treating hepatitis C virus infection, chronic hepatitis stays a severe medical health problem world wide with over 170 million infected people[3,14]. Even if treatment of chronic infected patients with pegylated interferon and ribavirin results in sustained virological response in 40 to 80%, depending on the genotype[22,39], there remains a large number of HCV positive patients, non-responders and relapsers. Until development of a vaccine HCV remains one of the most important diseases. The main problem in developing such a vaccine is the limited understanding of the type of immune response that is necessary for viral clearance and the occurrence of various genotypes and quasispecies of HCV, evolving rapidly under selection pressure by the immune response[2,61,64]. So most likely a vaccine must induce a broad immune response to clear HCV infection [4,26,60]. And indeed, humoral and cellular immune responses to several of the viral proteins have shown clearance of HCV infection in experimental settings[17,21, 32,51,63]. Dendritic cells (DC) are important players in the development of an adaptive immune response; specialized in antigen presentation and activation of T-cells. In addition, DC stimulate B-cells and natural killer cells[7,35,36,40], and produce cytokines such as IL-12 and IFN-α[44]. DC can induce either immunity or tolerance to hepatitis viruses [36,59]. It has been shown, that the function of DC is impaired in individuals who have become chronically infected with hepatitis viruses[10,11]. The interaction between chronic hepatitis viruses and DC is not clearly understood, however earlier studies have demonstrated reduced numbers of DC in the peripheral blood, reduced cytokine production, as well as a disruption of antigen presentation in patients who can not clear the virus[18,62]. This is thought to be one reason for the low virus specific T- and B-cell responses in chronic hepatitis patients. Interestingly, DC function and T-cell responses are restored in patients who clear the virus after a period of chronic infection, whether spontaneously or due to therapy[12,56]. Thus, it appears that stimulation and reactivation of DC could restore HCV specific immune response and help to eradicate the virus. Several approaches to use DC as cell based therapy are presently being studied[1,15,38,50]. Ex vivo generated and matured DCs therefore might be the most potent candidate for a cell based HCV vaccine. In fact, there are some promising results published for immunization with DCs for the human immunodeficiency virus and HCV [19,24,25]. Mouse DC can efficiently be activated in vitro using HBVsvp. Moreover, reinjection of such stimulated mouse DC into BALB/c mice led to strong and specific humoral and cellular immune responses in those mice, thus demonstrating that in vitro activated DC can effectively induce immune responses against HBV in vivo[20]. The DC must be in the correct maturation state to be sufficiently activated, which may be different regarding the focused target[43]. Besides the maturation state the inflammatory cytokine milieu and the area of origin (plasmacytoid or myeloid) seems to play an important role. Furthermore, a challenge is the site of injection. In some studies subcutaneous injected DC only migrated at low levels to the lymph nodes[52]. Reaching the lymph-node DC must show full ability to produce bioactive cytokines to properly activate T- and B-cells[29]. Many DC-based vaccines do not work due to these hurdles and the challenge is to find the right approach for the specific target.HCV pseudo-particles (HCVpp) were generated by assembling these full-length, unmodified E1 and E2 glycoproteins onto retroviral core proteins derived from murine leukeamia virus (MLV). Such pseudo-particles were obtained with E1E2 glycoproteins, derived from HCV genotypes 1a and 1b, that stand among the most prevalent and most resistant to interferon- therapy[66]. Retroviruses were chosen as platforms for assembly of HCVpp because their cores can incorporate a variety of different cellular and viral glycoproteins[53] and because they can easily transfer and integrate genetic markers into DNA of infected cells[42]. Chimpanzees immunized with recombinant HCV E1 and E2 showed protection against HCV infection[13]. Peptide immunizations have been successful in producing humoral and cellular immune responses[34,45]. But peptides do not deliver a great number of epitopes and are not foulded in the native protein form. To overcome these limitations virus like particles (VLP) have been created, consisting of both envelope proteins E1 and E2 using HCV core and retroviral packaging proteins, second named HCV pseudo particles (HCVpp)[8,9,41]. These particles mimic HCV virions in the best possible way and are therefore an interesting stimulant. We showed that HCVpp are able to activate mature murine DCs in vitro, making them an interesting tool to produce immune response and protection for HCV infection.
2. Materials and Methods
2.1. Cells and Culture Conditions
- Huh-7 human hepatocellular carcinoma cell line and HEK293T cells were cultivated in DMEM cell culture medium (Invitrogen, Germany), supplemented with 10% (v/v) fetal calf serum (FCS) (Invitrogen, Germany), 2 mM L-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml (Invitrogen, Germany). Bone marrow derived dendritic cells were maintained in RPMI 1640 medium (Invitrogen, Germany) supplemented with 10 % FCS, 2 mmol/L L-glutamine, 100 U/mL Penicillin, 100 mg/mL streptomycin and 50 μmol/L 2-mercaptoethanol (Invitrogen, Germany).
2.2. Production of HCV Pseudotype Particles
- To generate HCV pseudotype particles (HCVpp), HEK293T cells were transfected with expression vectors encoding the viral E1/E2 glycoproteins [phCMVDCE1 - E2(Con1)], human immunodeficiency virus (HIV)-Gag-Pol (pCMVDR8·74), and the packaging-competent green fluorescent protein (GFP)-containing retroviral transfer vector pHRCMV-Emd, which were kindly provided by Dr R. Bartenschlager, Department of Molecular Virology, University of Heidelberg[31,48]. For an expression control, we used the plasmid pcz vesicular stomatitis virus (VSV)-G instead of phCMVDCE1-E2(Con1). For transfection a calcium phosphate transfection kit was used (Clontech, Heidelberg, Germany)[8], according to the manufacturer’s instructions, using 8 μg of each plasmid (ratio of 1:1:1) with 62 μl 2 M CaCl2 and added sterile water to get 500 μl. Then we added the calcium-DNA mix drop wise to 500 μl of phosphate buffer. This was carried out under continuously vortexing the phosphate buffer. After an incubation period of 20 minutes, we added again drop wise the DNA crystals to plated cells with confluency of 70–80%. Medium change was done 16 hours later. Supernatants containing HCVpp were harvested 40–48 h after transfection, clarified by low speed centrifugation for 15 min, filtered through membranes with 0·45 mm pores and concentrated using Amicon Ultra-15 molecular filters with an exclusion size of 30 kDa (Millipore, Bedford, MA, USA).We usually concentrated the particles 20-fold and stored them at -80°C.
2.3. HCVpp Infection Assay
- Huh-7 human hepatocellular carcinoma cells were seeded the day before infection at 1 x 105 Huh-7 cells per well in a 12-well tissue culture plate (Greiner Labortechnik). One hour before infection, cells were washed three times with warm phosphate-buffered saline (PBS) and incubated with plain Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Karlsruhe, Germany). Then dilutions of viral supernatants containing the HCVpp were added to the cells and incubated for 3 h. The supernatants were removed and the cells incubated in DMEM supplemented with 10% (v/v) fetal calf serum (FCS) (Biochrom, Berlin, Germany) and 2 mm l-glutamine (Invitrogen) for 72 h at 37°C. The infectious titres, expressed as transducing units (TU)/ml, were determined as the percentage of GFP positive cells measured by fluorescence activated cell sorter (FACS) analysis using the formula analysis[(number of target Huh-7/volume of HCVpp)*(percentage of GFP-positive cells/100)]. Infected Huh-7 cells were trypsinized, suspended in PBS with 0·5% bovine serum albumin (Sigma-Aldrich) and analysed for GFP fluorescence by cytofluorometry.
2.4. Generation of Bone-marrow Derived Dendritic Cells (BMdDC)
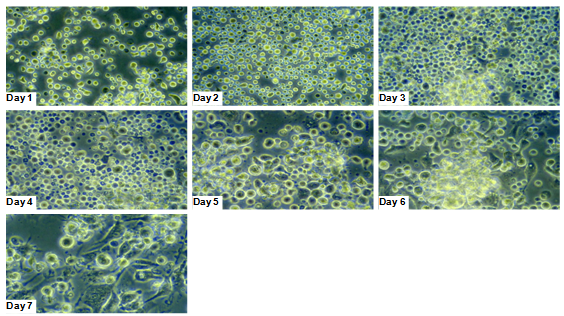
- For preparation of bone-marrow, we used 6–10 week old BALB/c mice, obtained from Charles River Breeding Laboratories (Sulzfeld, Germany). They were maintained under SPF conditions and handled according to international guidelines. After sacrificing the animals, the tibia and femur bones were used to prepare bone marrow cells. With minor adaptations, cultivation of bone-marrow cells was done following the Inaba protocol[27]. On day 7 we pooled non-adherent and loosely adherent cells. The isolated cell suspensions were either taken for FACS analysis or plated into 12 well culture plates (Greiner Labortechnik) at a density of 1.5×106 cells per well in one ml of complete BMdDC medium (Invitrogen, UK).
2.5. FACS-Analysis of BMdDC
- Flow cytometry analysis for measuring the expression of different surface molecules was performed with a FACSCalibur® cytometer and data was analyzed with Cell Quest Pro software (Beckton Dickinson). For staining, 2×105 cells were incubated in staining-buffer (PBS and 0.5% BSA, Invitrogen) with either 1 µl of specific antibodies or the corresponding isotype control (APC anti-mouse CCR-7 (Biozol, Eching), R-PE anti-mouse CD 11c, FITC anti-mouse CD 86, FITC anti-mouse MHC II (I-Ad) (all from BD Bioscience, Heidelberg)) for 30 min on ice in the dark. Stained cells were pelleted for 3 min at 2000 rpm (Biofuge pico; Kendro, Hanau) and were washed twice with staining-buffer. A negative control with unstained cells was run first to determine the baseline fluorescence. Checking for unspecific binding, marker-setting was done with isotype controls. For instrument settings and compensation of R-PE and FITC, samples stained with individual fluorescent probes were used.
2.6. Activating BMdDC with HCVpp
- On day 7, FACS analyses revealed 75.9% mature DC, which were placed in a 12-well culture plate with a concentration of 1.5×106 cells per ml. Twenty-four hours later, the DC were activated by adding nothing (negative control), HCVpp, 1 μg/ml E. coli lipopolysaccharide (LPS) (Sigma, St. Louis, MO), or HCVpp plus LPS together into the culture medium. Cells were harvested on day 9 and were washed extensively. Activation of DC was measured by FACS analyses.
3. Results
3.1. Production of HCVpp
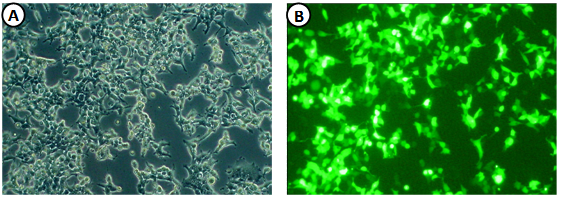
- HEK293T cells were transfected with expression vectors encoding the viral E1 and E2 glycoproteins, HIV retroviral core proteins and packaging-competent GFP-containing retroviral transfer vectors. HEK293T cells showed ~30-40% GFP positive cells 48 h after transfection indicating HCVpp production (Fig. 1). The recently developed HCV culture system enabled us to test the effect of infectious HCV particles on DC cells under controlled in vitro conditions.
3.2. Infectivity of HCVpp
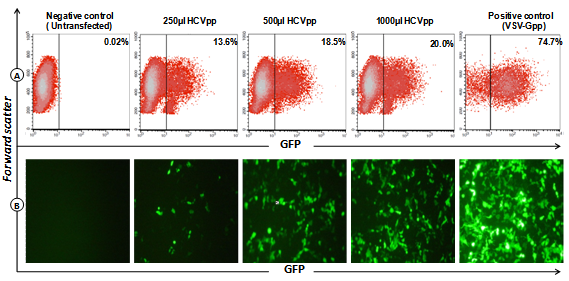
- Huh-7 human hepatocellular carcinoma cells were infected with HEK293T supernatants containing HCVpp or with VSV-G pseudotype particles as positive control, or left untransfected for negative control. Flow cytometry analysis showed 13.6–20.0% GFP-positive cells after infection with HCVpp-containing supernatant in different concentration (Fig. 2), demonstrating that the HCVpp preparation was indeed infective.
3.3. Flow Cytometry Analysis of Bone-marrow Derived Dendritic Cells (BMdDC)
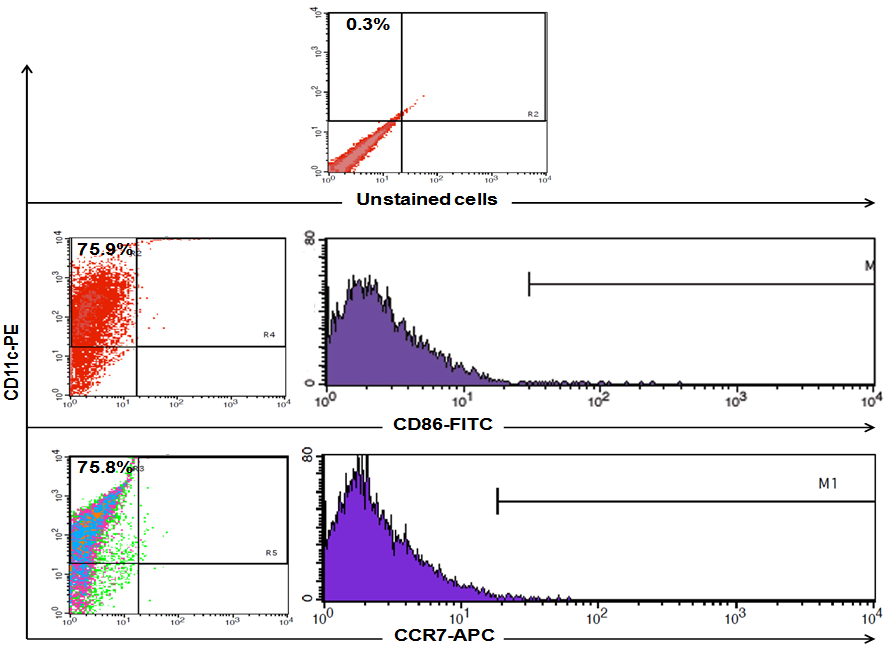
- To identify the differentiation of bone marrow towards the dendritic cell line, flow cytometry analysis of cultivated BMdDC was performed. Phenotype analysis of DC, identified by expression of CD11c showed 75.9% of the cultured cells were determined to be mature DC after 7 days of maturation with Granulocyte/macrophagecolony-stimulating factor (GM-CSF) and IL-4 (Fig. 3a, and 3b).
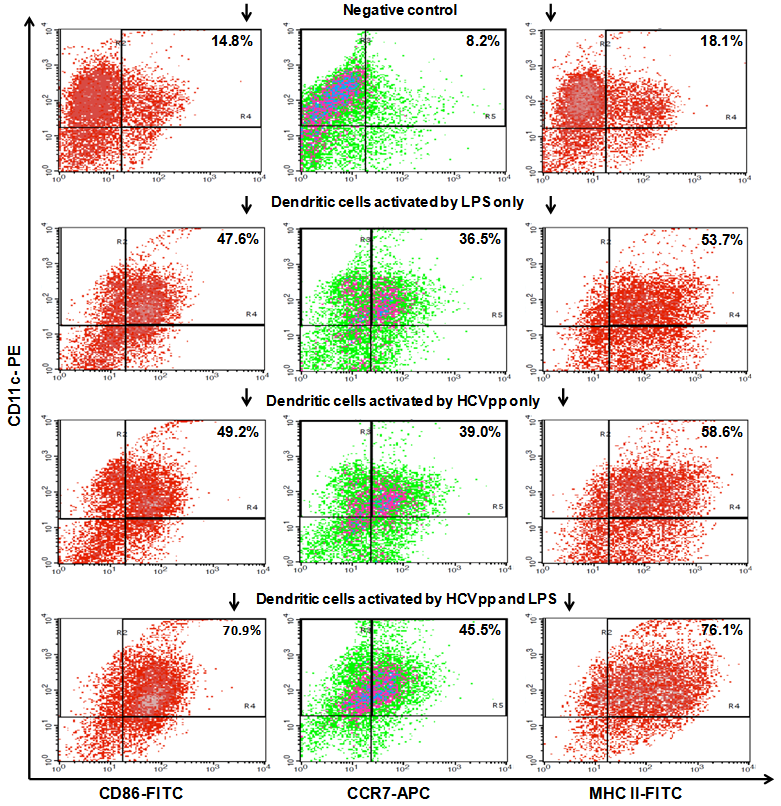
3.4. Dendritic Cells are Strongly Activated by HCVpp in Vitro
- As shown in Fig. 4 the DC in our study showed strong activation by HCVpp in vitro. Non-activated DC, specified by CD11c staining, presented CD 86 in 14.8%, CCR7 in 8.2%, and MHC II in 18.1%, in the FACS analysis. After 24 h of incubation with HCVpp and the co-stimulatory factor LPS these rates were increased to 70.9% for CD 86, 47.5% for CCR7, and 76.1% for MHC II, respectively. Incubation with LPS only leads to a middle alteration of the measured CD markers. Here CD 86 was found in 47.6%, CCR7 in 36.5% and MHC II in 53.7%, respectively. Incubation with HCVpp alone also resulted in an up-regulation of these markers, but lesser than found in the co-stimulated DC group. Here CD 86 was found in 49.2%, CCR7 in 39.0% and MHC II in 58.6%, respectively.
4. Discussion
- Chronic HCV infection may progress to chronic hepatitis, cirrhosis, hepatic failure, and hepatocellular carcinoma (HCC). HCV inhibits intracellular interferon pathways, impairs DC activation and T-cell responses[6,30,54]. In addition, it induces a state of T-cell exhaustion and selects escape variants with mutations in immunodominant T-cell epitopes[60]. This is especially important since the clearance of HCV infection requires strong and broadly cross-reactive T-cell and neutralizing antibody responses[37,46,55]. It has been shown that the development of a multi-specific T-cell response during acute HCV infection is associated with spontaneous clearance of infection[57]. A successful immune response against HCV is therefore based on sufficient innate and adaptive immune responses. However, although B and T lymphocytes respond to antigens with high specificity, they are by themselves not capable of making the complex decisions of immune activation. Induction of an effective immune response requires the participation of professional host antigen presenting cells (APC), the most potent of which are DC. They play a key role in the initiation of immune responses to foreign antigens. Their antigen uptake and presentation capacities enable them to prime and activate T cells[7,58]. The function of DC differs according to their localization and level of maturation. In general these cells are strong stimulators of the allogenic mixed leukocytes reaction, induce antigen specific humoral and cellular immune responses, produce and induce a variety of cytokines of both Th1 and Th2 types, ensure the survival and activity of the antigen-specific lymphocytes including CTL and induce immunogenic tolerance[7,49]. HCV has been shown like other viruses (e.g. herpes simplex virus, measles virus) to affect and to impair DC function. DC derived from HCV patients and from patients with HCC in a HCV cirrhotic liver showed impaired abilities to stimulate allogenic T cells and to produce IFN-γ[6,28,30]. Monocyte derived DC from patients with chronic infection fail to respond to maturation stimuli. Instead they maintain their immature phenotype, reflected by their pattern of cell surface markers and by their continued capacity to uptake antigen. Moreover, DC from patients who have resolved HCV infection behaves like DC from healthy donors: in response to maturation stimuli, they decrease antigen uptake, up-regulate expression of appropriate surface marker, and are potent stimulators of allogenic T cells[5]. In vitro activation of DC followed by immunization with these DC leads to the induction of strong and specific antibody and T-cell responses in the hepatitis B context[20]. For HCV it has been shown that activation of DC by the core or the NS3 protein leads to maturation and stimulation of T-cells[33]. In addition, it was shown that DC function was restored in chronic HCV infected patients by the use of IL-10 inhibitors[16]. Thus, re-activation of DC may be an important tool in fighting HCV infection. In the present study, we were able to demonstrate that mouse DC can efficiently be activated in vitro using HCVpp, demonstrated by FACS analysis showing increased CD86, MHC II, and CCR-7.HCVpp were chosen to activate the DC for several reasons. They contain the E1 and E2 proteins and present them as closely to mature virions as possible. Due to that, neutralizing epitopes of the E1 and E2 proteins are potentially presented in the natural three-dimensional fashion. Here, we have successfully generated infectious pseudo-particles that were assembled by displaying unmodified and functional HCV glycoproteins onto retroviral and lentiviral core particles. The presence of a green fluorescent protein marker gene packaged within these HCV pseudo-particles allowed reliable and fast determination of infectivity mediated by the HCV glycoproteins. Primary hepatocytes as well as hepato-carcinoma cells were found to be the major targets of infection in vitro. High infectivity of the pseudo-particles required both E1 and E2 HCV glycoproteins. In addition, these pseudo-particles allowed investigation of the role of putative HCV receptors. HCVpp may mimic the early infection steps of parental HCV and will be suitable for the development of much needed new antiviral therapies.
- DC were chosen since we and others showed that they can be used to strongly induce immune responses which exceed the responses achieved by immunization with proteins or peptides only[20]. There are many challenges to face using DC as a therapeutic vaccine. The DC must be in the correct maturation state to be sufficiently activated, which may be different regarding the focused target[43]. Early used DCs were immunogenic, but suboptimal with regard to their lymph-node homing ability and T-cell stimulatory potential[29]. Besides the maturation state the inflammatory cytokine milieu and the area of origin (plasmacytoid or myeloid) seems to play an important role. Furthermore, a challenge is the site of injection. In some studies subcutaneous injected DC only migrated at low levels to the lymph nodes[52]. Reaching the lymph-node DC must show full ability to produce bioactive cytokines to properly activate T- and B-cells[29]. Many DC-based vaccines do not work due to these hurdles and the challenge is to find the right approach for the specific target.LPS was chosen because it has been shown potential in vaccinating, easy storage and wide application, with both carrier and adjuvant functions that activate DC[23]. It was used in animal models and humans and can be considered save. In addition, the use of a non-infectious adjuvantic stimuli, like LPS, is probably at lower risk than the activation of DC by (adeno-)viral vectors[50]. It is well known, that LPS is used as co-stimulatory factor for DC[65], adjuvantic immune stimuli strongly enhance immune responses and may be indispensible for significant in vitro activation of mononuclear cells[38,47].In conclusion, our data in the present study demonstrate that HCVpp were successfully generated and assembled by displaying unmodified and functional HCV glycoproteins onto retroviral and lentiviral core particles. We were able to demonstrate that mouse DC can efficiently be activated in vitro using HCVpp, identified by FACS analysis showing increased CD86, MHC II, and CCR-7. However, the effect was much higher when HCVpp pulsed DC was done in presence of the co-stimulance LPS, which was needed to significantly increase DC activating surface markers. We believe that use of DC as a cellular based therapy is of great interest and should be evaluated further to sufficiently fight chronic HCV infection. DC function of HCV patients could be restored in vitro, possibly assisting the patients to better control or even clear the virus. Vaccination with ex vivo activated DC may be a promising tool for therapeutic or prophylactic vaccine against the hepatitis C virus.
ACKNOWLEDGEMENTS
- We thank Professor Dr. Rolf Bartenschlager, University of Heidelberg and his group for their kind gift of HCV expression plasmids.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML