-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Virology and Molecular Biology
p-ISSN: 2163-2219 e-ISSN: 2163-2227
2012; 1(3): 28-34
doi: 10.5923/j.ijvmb.20120103.02
Isolation and Characterization of a Nucleopolyhedrovirus from Rachiplusia nu (Guenée) (Lepidoptera: Noctuidae)
Vanina A. Rodríguez 1, Mariano N. Belaich 1, Graciela Quintana 2, Alicia Sciocco Cap 2, Pablo D. Ghiringhelli 1
1Departamento de Ciencia y Tecnología, LIGBCM-AVI, Roque Sáenz Peña 352, Bernal, 1876, Argentina
2INTA, IMyZA-CCVyA, CC25, Castelar, 1712, Argentina
Correspondence to: Pablo D. Ghiringhelli , Departamento de Ciencia y Tecnología, LIGBCM-AVI, Roque Sáenz Peña 352, Bernal, 1876, Argentina.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Rachiplusia nu (Guenée) (Lepidoptera: Noctuidae) is an important pest of vegetable crops widely distributed in South America. At present, its control is based on the use of broad spectrum chemical insecticides. In order to develop a more selective alternative to be applied in integrated or organic pest management programs, we isolated and characterized a baculovirus that infects R. nu, denominated RanuMNPV (Rachiplusia nu multiple nucleopolyhedrovirus) in view of its morphology and pattern of infection. According to optical microscope images of in vitro infected cell cultures, data from bioassays carried out with various species of larvae, and DNA sequences from p74, polyhedrin, v-cathepsin, v-chitinase, lef8 and lef9 genes, this isolate could be considered as a variant of Autographa californica MNPV with a different host range.
Keywords: RanuMNPV, AcMNPV, Biological Control, Host Range
Cite this paper: Vanina A. Rodríguez , Mariano N. Belaich , Graciela Quintana , Alicia Sciocco Cap , Pablo D. Ghiringhelli , "Isolation and Characterization of a Nucleopolyhedrovirus from Rachiplusia nu (Guenée) (Lepidoptera: Noctuidae)", International Journal of Virology and Molecular Biology, Vol. 1 No. 3, 2012, pp. 28-34. doi: 10.5923/j.ijvmb.20120103.02.
Article Outline
1. Introduction
- The family Baculoviridae comprises a large number of viruses which infect arthropods, mostly insects from the order Lepidoptera and some others from Hymenoptera and Diptera[1],[2]. The viral genome is double stranded circular DNA, with sizes ranging from 80 to 180 kbp. Mature virions, assembled late in the infectious cycle, are surrounded by a crystalline proteinaceous matrix. These structures are known as occlusion bodies (OB), and their morphology and size have been used to subdivide the Baculoviridae into two genera: nucleopolyhedrovirus (NPV) and Granulovirus (GV). More recently, on the basis of genome analysis and phylogenetic studies, a new classification and nomenclature was proposed and accepted. The updated classification includes four genera: Alphabaculovirus (lepidopteran-specific NPV),Betabaculovirus (lepidopteran-specific GV), Gammabaculovirus (hymenopteran-specific NPV) and Deltabaculovirus (dipteran-specific NPV)[3]. Viruses grouped within the genus Alphabaculovirus produce OBs constituted by polyhedrin as the major matrix protein and several enveloped virions containing a single (SNPV) or multiple (MNPV) nucleocapsids. Alphabaculovirus is further divided into Group I or Group II, considering sequence analyses and the presence of gp64 gene[4].Among the NPVs isolated from noctuid larvae of the Subfamily Plusiinae, many have demonstrated a great potential as biopesticides and are successfully used in pest management programs[5-11]. Studies based on restriction endonuclease mapping, nucleic acid hybridization, and complete or partial sequencing of MNPVs isolated from Autographa californica (AcMNPV), Rachiplusia ou (RoMNPV) and Anagrapha falcifera (AfMNPV), revealed the existence of a close genomic relationship among them[12-15]. For that reason, RoMNPV and AfMNPV were proposed as variants of AcMNPV[1], although they present differences in host range and virulence[16].The looper Rachiplusia nu (Guenée, 1852) (Lepidoptera: Noctuidae, Plusiinae), is a polyphagous leaf-feeder species widely distributed in South America. Larvae cause damage in several economically important species, such as sunflower, tobacco, soybean and vegetable crops. At present, the management of R. nu is based on the applications of broad spectrum chemical insecticides. For many years, studies have been focused on the finding of alternative biological control methods. In this regard, isolates of NPVs from R. nu were evaluated under laboratory and field conditions[17-19]. However, none of them were fully characterized.With the objective of proposing a bio-pesticide for the control strategy of R. nu, we isolated and characterized a Rachiplusia nu nucleopolyhedrovirus in terms of its major biological, morphological, and biochemical properties. The relationship among this isolate (named RanuMNPV), AcMNPV and other genomic variants of the baculovirus prototype are also discussed.
2. Materials and Methods
2.1. Larval rearing and Baculovirus Production
- Larvae of Rachiplusia nu were obtained from a colony established in our laboratory (IMyZA-INTA), and maintained on artificial diet at 25 ± 1℃, 14:10 h light-darkness (LD) cycle, and 60% relative humidity (RH)[20]. The virus described in this study was isolated from diseased Rachiplusia nu larvae field collected in Oliveros (Santa Fe Province, Argentina). The virus was propagated in the host by infecting early fourth-instar larvae by diet surface contamination (1,500 OBs/mm2). OBs were purified from aqueous homogenates of dead larvae by two rounds of centrifugation in continuous 40-65% (wt/wt) sucrose gradients, at 100,000 g for 1 h at 4℃. The OB-containing fraction was collected, diluted with distilled water, and concentrated by centrifugation at 8,000 g for 20 min, at 4℃. The pellets were resuspended in sterilized double-distilled water and conserved at -20℃. The quantifications of OBs were performed by calculating the mean of five independent counts using hemocytometer (Neubauer chamber) and optic microscopy (Nikon Eclipse TS 1000).
2.2. Morphological Studies
- Purified OBs from infected R. nu were embedded in 2% (wt/v) agarose and fixed in 3% (v/v) glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) - 0.25 M sucrose for 3 h. Post-fixation was carried out for 2 h in 1% osmium tetroxide in 0.1 M cacodylate buffer. Then, samples were embedded in Epon-Araldite after being dehydrated through an ethanol-propylene oxide series. For phase contrast light microscopy, sections were cut 1 μm thick and examined unstained. For transmission electron microscopy, sections about 70 nm thick were stained in 5% (v/v) aqueous uranyl acetate for 30 min, lead citrate for 10 min, and examined with a JEM-1200 EX II electron microscope.
2.3. Recovery of Budded Virions for in Vitro Assays
- Purified OBs from infected R. nu were used for genomic DNA isolation. Briefly, virions were released in 0.1 M Na2CO3, 0.17 M NaCl, 0.01 M EDTA (pH 10.5) at 37℃, for 30 min. After this, the suspension was neutralized with 1 N HCl and undissolved polyhedra were removed by low–speed centrifugation. Then, 0.5% (wt/v) SDS and proteinase K (0.25 mg/ml) were added, incubating at 37℃ for 3 h. Finally, DNA was isolated by phenol extraction and concentrated by ethanol precipitation[21].Spodoptera frugiperda (Sf9) cells[18, 37] were grown at 27 ℃ in GRACE’s medium (Invitrogen) containing 10% fetal bovine serum (Bioser) and supplied with antibiotics and antimycotics (Invitrogen). To produce budded viruses (BV) of R. nu baculovirus, a total of 1 x 106 Sf9 cells were seeded in 60 mm plate and incubated at 27℃ for 1 h. Six microliters of CellFECTINTM (Gibco Life Technologies) were diluted in 100 µl of serum-free GRACE's medium, mixed with viral DNA (1-2 µg diluted in 100 µl of serum-free GRACE'S medium), incubated at room temperature for 40 minutes and diluted with 800 µl of serum-free GRACE's medium. After removing the culture medium, the DNA/CellFECTIN mix was added to the cell monolayers and incubated at 27℃ for 5 h without agitation. Subsequently, 2 ml of GRACE'S medium with 10% fetal bovine serum were added and the cells incubated at 27℃. Seven days post-transfection the conditioned media containing BVs were collected, centrifuged for cell debris clarification and stored at 4℃ until use. Two rounds of virus amplification were performed using semi-confluent Sf9 cell monolayers and recovery BVs[multiplicity of infection (moi): 0.1].
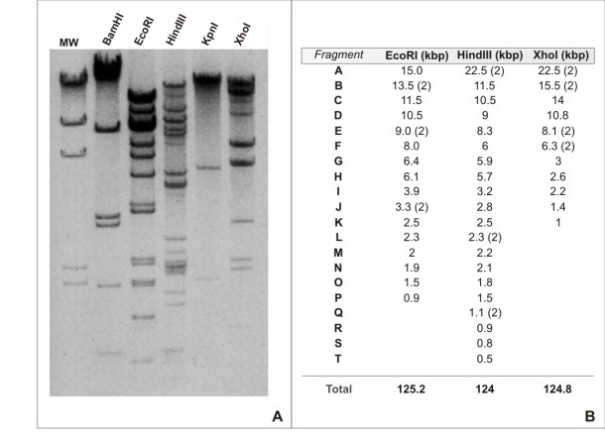
2.4. Viral Genome Size Determination
- Sixty percent confluent monolayers of Sf9 cells growing in previously mentioned culture conditions were infected with R. nu baculovirus, (moi: 0.1) and incubated for 7 days at 27℃. Then, BVs were collected from conditioned media, centrifuged for cell debris clarification and resuspended in lysis buffer (10 mM Tris-HCl, 10 mM EDTA, 0.25% SDS). After a 37℃ overnight treatment with proteinase K (20 mg/ml), virus DNAs was purified by standard phenol/ chloroform extraction and ethanol precipitation[21]. The DNA was quantified by spectrometry (OD260, SmartSpec 3000 BioRad) and was tested by electrophoresis in 0.6% (wt/v) agarose gel and ethidium bromide staining. The R. nu baculovirus genome size was estimated through restriction analysis using BamHI, EcoRI, HindIII, KpnI and XhoI (Invitrogen) and subsequent agarose gel electrophoresis using appropriate molecular weights as references (PB-L).
2.5. Cloning and Partial Sequencing of Polyhedrin, p74, v-cathepsine, v-chitinase, lef8 and lef9 genes
- With the goal to classify R. nu baculovirus, some auxiliary and core genes were partially sequenced. Thus, aliquots of R. nu baculovirus genome were independently digested using HindIII and EcoRI restriction endonucleases (Invitrogen), pZErO 2.0 (Invitrogen) was selected as molecular cloning vector, digested with the same enzymes, and dephosphorylated using alkaline phosphatase (Invitrogen). Then, R. nu baculovirus restriction endonuclease fragments were cloned using T4 DNA ligase (Invitrogen) and Escherichia coli Top 10 (Invitrogen). Later, the HindIII and EcoRI genome libraries were screened to detect polyhedrin and p74 genes by Dot blot assays (BioRad). To find the polyhedrin locus a heterologous probe deriving from polyhedrin orthologous gene of AgMNPV was used[25]. To find p74, the probe was based on a PCR product derived from of R. nu baculovirus p74 gene obtained using Taq DNA polymerase (PB-L) and universal primers[26]. Both fragments were purified using QIAEX II Gel extraction Kit (QIAGEN), labeled using alkaline phosphatase, and the hybridizations were performed according to the manufacturers´ specifications (AlkPhos direct, Genes Images from Amersham Pharmacia Biotech). Moreover, fragments of v-cathepsin, v-chitinase, lef8 and lef9 genes were amplified by PCR using Taq DNA polymerase and the primers p_cat398/p_cat896, p_quit68 / p_quit1513, prL8-1/prL8-2 and prL9-1/prL9-2, repectively; the reaction conditions used were the previously described[27],[28]. After this, amplicons were cloned in pGemT-easy vector (Promega). All molecular clones containing fragments of the six genes tested were recovered and the inserts sequenced using universal primers (Macrogen, http://www.macrogen.com).
2.6. Sequence Analyses
- Sequence analyses were performed using available genome sequences of baculoviruses(www.ncbi.nlm.nih.gov). Multiple alignments were obtained with ClustalX program, using Gap opening 10 and Gap extension 0.1 as parameters for pairwise alignments, and Gap opening 10 and Gap extension 0.05 for multiple alignments. Dayhoff distance corrected UPGMA distance analyses (gamma shape parameter: 2.25; gaps/missing data: complete deletion, respectively) and phylogenetic trees (1000 bootstrap replicates) were inferred from the amino acid sequence alignments by using MEGA, version 4.0[29],[30]. The pairwise comparisons of amino acid sequences were performed using ClustalW with the default parameters. Distance matrices from aligned nucleotide sequences were determined by using the Pairwise Distance calculation of the same software applying the Kimura 2-parameter (K-2-P) model[30].
2.7. Larval Bioassays
- Rachiplusia nu pre-molted second-instar larvae were placed into individual wells (1 cm3) of a 50 multiwell device containing artificial diet (without formalin) contaminated on surface with R. nu baculovirus´s OBs (2, 8, 32, or 128 OBs/mm2). Then, larvae were incubated at 25 ± 1℃, 14:10 LD and 50% RH. Mortality was recorded daily until pupation. Three replicate assays of 50 individuals each one were carried out for the different tested doses. Other 50 additional individuals were used as controls. The concentration-mortality response data were analyzed by probit[22] using MSTAT-C, and indicated as LC50 (Lethal Concentration 50) values. For comparative purposes, equivalent bioassays were performed against two major regional soybean pests: Anticarsia gemmatalis and Spodoptera frugiperda (Lepidoptera: Noctuidae). Thus, fifty third-instar larvae were individually placed into 40 ml plastic cups that contained surface contaminated diet (3,000 OBs/mm2); while 30 individuals were used as controls. In any case, mortality by baculovirus infection was verified by optic microscopy examination.
2.8. In Vitro Cell Culture Host Range
- BVs of R. nu baculovirus and AcMNPV were amplified and tittered by plaque assay in Sf9 cells[23]. The virus infectivity in Sf9, Sf21 (both derived from Spodoptera frugiperda), Hi-5 (derived from Trichoplusia ni) and UFL-Ag-286 (derived from Anticarsia gemmatalis)[24] cell lines were tested. To accomplish this, cells growing under previously mentioned conditions at 60% confluence in 24 multiwell plates (Sarstedt) were exposed with R. nu baculovirus or AcMNPV (moi: 0.5). After incubating during 4 days, cells were photographed using an optic microscope (Nikon Eclipse TE2000) and the conditioned media harvested in order to quantify BV productions by a colorimetric assay. Briefly, Sf9 cells growing in 96 multiwell plates were treated with the virus samples and 4 days incubated. Then, cells were fixed using 10% (v/v) methanol for 10 minutes, and stained by 0.1% (wt/v) violet crystal treatment. Finally, absorbance measures at 570 nm (Dynex Technologies MRX tc Microplate Reader) were performed and relativized respect to previously plaque assay tittered AcMNPV and R. nu baculovirus stocks.
3. Results and Discussion
3.1. Morphological Studies of RanuMNPV
- R. nu infected larvae with a field isolated virus were studied to analyse some morphological properties. Thus, electron microscope examination of ultrathin sections revealed the presence of OBs with typical polyhedron morphology that averaged 1.52 µm (+/- 0.3) in diameter. Each occlusion body contained several virions with one to eight nucleocapsids (Figure 1). These data are in agreement with the virus description for multiplenucleopolyhedroviruses (MNPVs) of Lepidoptera[2],[3]. Consequently, the Rachiplusia nu virus isolate was denominated RanuMNPV (Rachiplusia nu multiple nucleopolyhedrovirus).
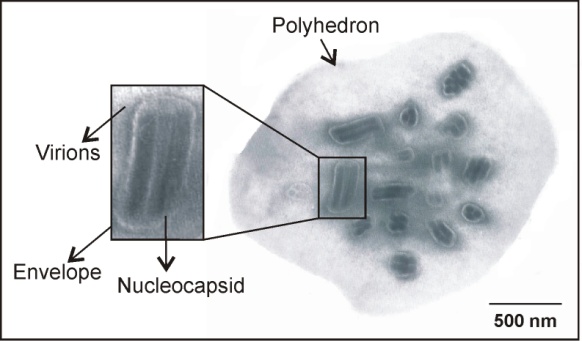 | Figure 1. Electron micrograph of an occlusion body of RanuMNPV, showing longitudinal and cross sections of virions containing multiple nucleocapsids. Bar: 500 nm |
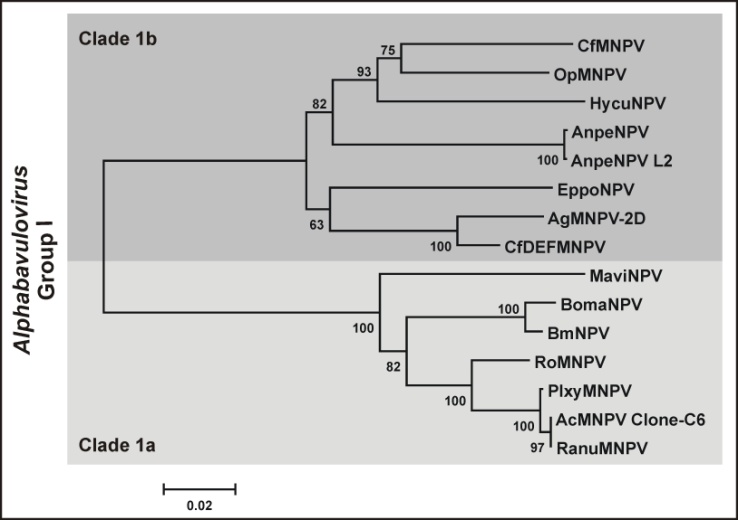
3.2. RanuMNPV Genome Analysis and Classification
3.3. Host Range Studies
- Considering previous data and with the aim to characterize the RanuMNPV host range, larval bioassays in various lepidoteran were carried out. Thus, the LC50 of RanuMNPV in Rachiplusia nu larvae was 10.4 (range: 8.5 to 16.2) OBs/mm2. In contrast, Spodoptera frugiperda larvae were not susceptible to RanuMNPV under the tested conditions, while 100% mortality was obtained against Anticarsia gemmatalis larvae considering percentage of mortality and disease development as criteria of susceptibility. In view of that, new bioassays using doses from 8 to 2,048 (rate: 4) OBs/mm2 were carried out against Anticarsia gemmatalis, allowing to estimate a value of 410 OBs/mm2 as LC50. This result was different to previous reports, suggesting that RanuMNPV had a differential host range respect to AcMNPV[34].
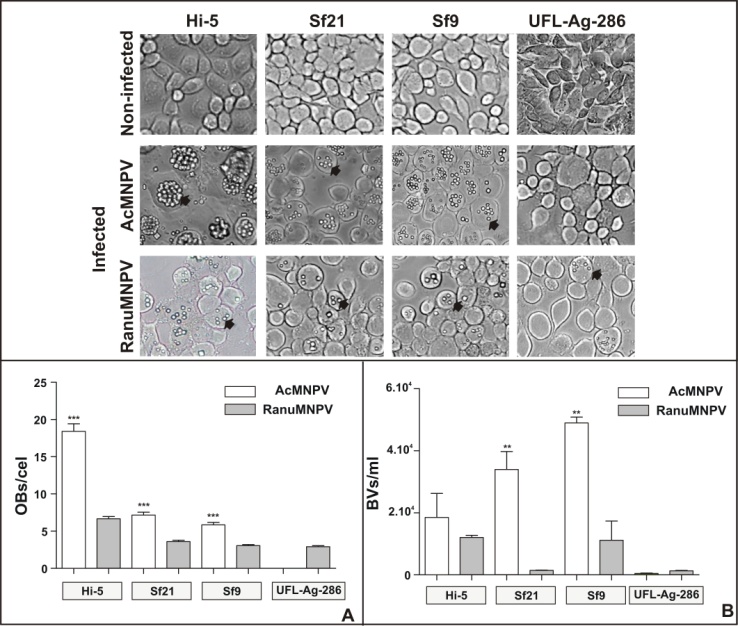
- Moreover, to verify the infectivity of RanuMNPV BVs in in vitro cell cultures various infection assays in Sf9, Sf21 (both from Spodoptera frugiperda), Hi-5 (from Trichoplusia ni) and UFL-Ag-286 (from Anticarsia gemmatalis) cells were performed. Four days post- inoculation, cells were analyzed by light microscopy (Figure 4A). All tested cell lines exposed to RanuMNPV showed a typical cytopathic effect, even UFL-Ag-286; this was not observed when AcMNPV was used, which clearly showed presence of OBs in Sf9, Sf21 and Hi-5 cells but not into the A. gemmatalis derived cell line. Specially, the numbers of OBs per infected cell produced by AcMNPV and RanuMNPV were significantly different for all tested cell lines, suggesting that the new isolate had a particular pattern of infection and productivity (p<0.0001) (Figure 4.B. and C.). In all tested cases, higher BV productivities were observed when cells were infected with AcMNPV, only with the exception of RanuMNPV in UFL-Ag-286 cells. Probably, the detected differences in the capabilities of these NPVs to establish productive infections in tested cell lines may be due to the presence or absence of cellular or viral factors necessary for genome replication, assembly and/or virus budding.Despite the extremely high sequence similarity found between RanuMNPV and AcMNPV, the experimental host ranges studied in laboratory conditions revealed to be significantly different suggesting that other non detected sequence variations occur.
4. Conclusions
- The isolation and partial characterization of a baculovirus obtained from a single diseased Rachiplusia nu larva is described by this work. The structural and molecular studies showed the presence of Multiple Nucleopolyhedrovirus (MNPVs) into the cell nuclei of affected tissues that contained nucleocapsids carrying genomes with a size estimated in ≅ 125,000 bp. According to the accepted strategy to assign species and genus for new baculovirus isolates[27],[28], total (p74 and polyhedrin genes) and partial (v-cathepsine, v-chitinase, lef8 and lef9 genes) sequences were obtained and analyzed. Thus, the Kimura 2 parameter model was applied showing that RanuMNPV would be considered as a new variant of AcMNPV species, belonging to the Clade 1a of Group I Alphabaculovirus genus. Despite this classification, the performed biological assays revealed a different behavior between AcMNPV and RanuMNPV, consequently exposing a dissimilar host range. Probably, the standard baculovirus species assessment should be reconsidered in future, involving the comparisons of more conserved sequences and including some phenotypic factors such as structural dimensions and host range. Although more evidences are needed, considering our results it is possible to speculate that the differences in p74 gene be important in the larva infectivity. That protein is an important per os infectivity factor and possibly few amino acid changes may be useful and necessary to support primary infections in new hosts. However, other variations in RanuMNPV genome should be occur because BV forms supported the infection into A. gammatalis derived cell lines, multiplication cycle that not require of viral factors crucial for the late baculovirus phenotype. For these reasons, the complete genome sequence of RanuMNPV appears as the next step to complete its characterization, and thus understand what genotypic differences may be crucial in host infectivity.This report shows the versatility and variability of AcMNPV and its variants in nature, and provides information that can be applied to optimize the use of baculoviruses as biological control agents of agriculture pests. In particular, RanuMNPV is a new baculovirus isolate that may be proposed as an alternative to control Rachiplusia nu larvae. Besides, this virus adds another useful candidate which together with AgMNPV can attend in controlling Anticarsia gemmatalis, one of the most important pests in South America.
ACKNOWLEDGEMENTS
- This work was supported by research funds from Agencia Nacional de Promoción Científica y Técnica (ANPCyT), Universidad Nacional de Quilmes and Instituto Nacional de Tecnología Agropecuaria (INTA). PDG and MNB are members of the Research Career of CONICET (Consejo Nacional de Ciencia y Tecnología); ASC and GQ are research members of INTA. The authors thank Dr. Bergmann Morais Ribeiro (University of Brasilia, Brasil) for providing RoMNPV, and UFL-Ag-286 cells, respectively.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML