-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Tumor Therapy
p-ISSN: 2163-2189 e-ISSN: 2163-2197
2020; 9(1): 5-11
doi:10.5923/j.ijtt.20200901.02

KIM-1 as an Early Predictive Marker for Cyclosporine A Nephrotoxicity in Leukemic Patients Performing Peripheral Stem Cells Transplantation
Hend Essam Abd El-Ftaah 1, Ola Sayed M. Ali 1, Raafat M. Abd El-Fattah Soliman 2, Noha Abdel-Rahman Eldesoky 1
1Biochemistry Department, Faculty of Pharmacy (Girls), Al-Azhar University, Cairo, Egypt
2Hematology and Bone Marrow Transplantation Department, Faculty of Medicine, Cairo University, Cairo, Egypt
Correspondence to: Noha Abdel-Rahman Eldesoky , Biochemistry Department, Faculty of Pharmacy (Girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

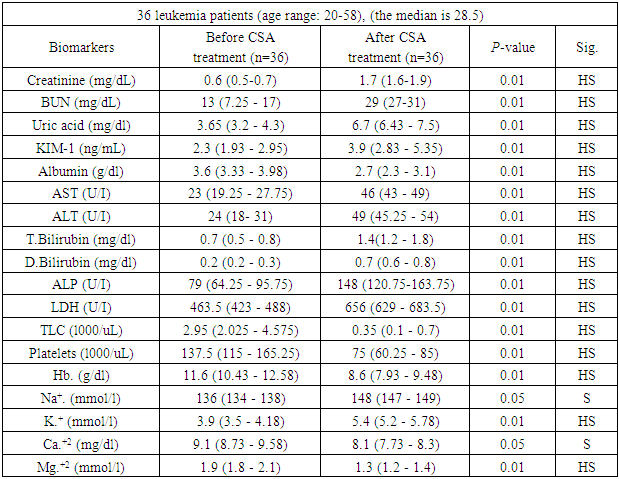
Background: Kidney injury molecule-1 (KIM-1) is a transmembrane glycoprotein related to acute kidney injury. Its role in the prediction of cyclosporine A (CSA) nephrotoxicity is unclear and up to our knowledge, this is the first time to investigate its usage after peripheral stem cell transplantation (SCT) as established marker of tubulointerstitial injury. Objective: to explore the usefulness of KIM-1 for early identification of CSA nephrotoxicity in Egyptian leukemic patients performing peripheral SCT. Methods: This study is a cross sectional study that was carried out on 36 leukemic patients performing SCT. Samples were collected from patient before the start of CSA treatment (Just after SCT) and after 14 days of CSA treatment. Serum KIM-1 was measured by ELISA, and CSA was measured by using automated clinical chemistry analyzer. Serum creatinine, blood urea nitrogen, uric acid, serum albumin, ALT & AST, bilirubin, ALP, LDH, sodium, potassium, calcium and magnesium levels were measured by VITROS 5600/XT 7600 Integrated Systems. Complete blood count was measured by fully automated hematology cell counter. After follow up of patients for 32 days, 12 of them developed nephrotoxicity so the patients were further divided according to nephrotoxicity status. Results: Serum level of KIM-1 was significantly higher after CSA treatment than before (p≤0.01). KIM-1 showed significant differences between positive and negative nephrotoxicity groups regarding before, after CSA and ∆Change values (p≤0.01, p≤0.01, p≤0.02) respectively. Whereas the only significant difference obtained regarding traditional kidney markers, was creatinine after CSA treatment (p≤0.02). Conclusion: Serum KIM-1 is superior to other kidney markers including creatinine, BUN and urea in the early prediction of CSA nephrotoxicity.
Keywords: Stem cell transplantation, Leukemia, Cyclosporine A, Nephrotoxicity, KIM-1
Cite this paper: Hend Essam Abd El-Ftaah , Ola Sayed M. Ali , Raafat M. Abd El-Fattah Soliman , Noha Abdel-Rahman Eldesoky , KIM-1 as an Early Predictive Marker for Cyclosporine A Nephrotoxicity in Leukemic Patients Performing Peripheral Stem Cells Transplantation, International Journal of Tumor Therapy, Vol. 9 No. 1, 2020, pp. 5-11. doi: 10.5923/j.ijtt.20200901.02.
Article Outline
1. Introduction
- Hematopoietic cell transplantation is a potentially curative treatment for many patients with high-risk hematologic malignancies, as well as a variety of other hematopoietic, immune, metabolic, and malignant diseases [1]. It is well known that immunosuppression is required after transplantation to prevent episodes of graft rejection and subsequently to reduce morbidity and mortality [2]. CSA is a first-line immunosuppressant for anti-rejection therapy after solid organ transplantation [3]. CSA nephrotoxicity was discovered early after its first use as a marked unexpected nephrotoxicity that had not been observed during animal experiments [4].Early detection of CSA nephrotoxicity is critical for preventing irreversible kidney injury [5] and CSA blood levels give little help in predicting kidney injury development [6]. The most widely used clinical standard for diagnosis of CSA nephrotoxicity is serum creatinine. When serum creatinine increases, it reflects a significant impairment of the glomerular filtration rate (GFR) [7]. However, it is not a sensitive and specific index since serum creatinine assesses renal function loss and changes in filtration capacity rather than kidney tissue injury [8]. Also, serum creatinine level is greatly affected by race, muscle mass, age, gender, hydration and protein intake [9].CSA level and kidney markers are usually monitored twice per week throughout the course of treatment and the dose of CSA is reduced if any kidney affection is observed. However, in spite of the precise monitoring of CSA and kidney markers levels, lots of patients develop kidney failure [10]. Hence arises the need for a new biomarker that could catch the early onset of tubular injury even before traditional kidney markers change and not affected by other interfering factors.KIM-1 (also known as TIM-1—T-cell immunoglobulin and mucin-containing molecule) was originally discovered during a screen for molecules involved in the AKI pathogenesis [11]. It is a member of the immunoglobulin gene superfamily. Structurally, KIM-1 closely resembles mucosal addressin cell adhesion molecule-1 [12].KIM-1 is a promising biomarker because its expression is normally low, but highly upregulated in proximal tubular cells after kidney injury both in rodents and in humans [13]. The ectodomain of KIM-1 shed into the lumen is mediated by matrix metalloproteinases with the aid of mitogen-activated protein (MAP) kinase activation, thus could serve as a blood biomarker of kidney injury with sensitive results in plasma and serum in mice, rats, and humans [14].So, the aim of the present study is to estimate the serum concentration of KIM-1 in leukemic patients who administer CSA after bone marrow transplantation to explore its usefulness for the early identification of cyclosporine A-induced nephrotoxicity.
2. Subjects and Methods
2.1. Study Design
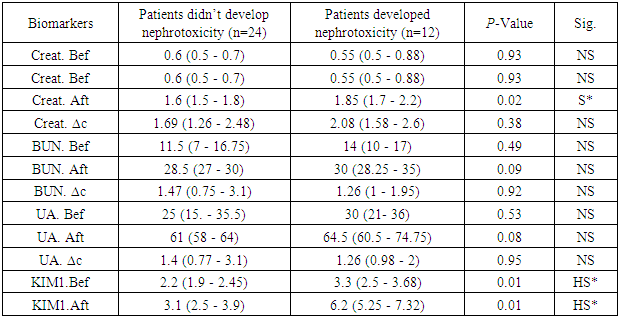
- This study is a cross sectional study that was carried out on 36 leukemic patients recruited from Nasser Institute Hospital in the period from July 2017 to June 2018. All patients were subjected to SCT followed by CSA administration to prevent allograft rejection. Samples were collected from patient as follow:1. Before the start of CSA treatment (Just after SCT).2. After 14 days of CSA treatment. Leukemic patients were further categorized into: Acute lymphocytic leukemia (ALL) patients (n=10), Acute myeloid leukemia (AML) patients (n=20) and chronic myeloid leukemia (CML) patients (n=6). After follow up of patients for 32 days, 12 of them developed nephrotoxicity and 24 didn’t develop nephrotoxicity. Nephrotoxicity was determined by increased urea and creatinine levels, oliguria less than 500 ml per day and/or hematuria [15].So, the data of the patients were further divided into 2 groups according to nephrotoxicity status and a comparison was done between both groups regarding the performance of kidney markers in prediction of kidney status. The present study conforms to the Declaration of Helsinki, US Federal Policy for the Protection of Human Subjects, and European Medicines Agency Guidelines for Good Clinical Practice and was approved by Research Ethical Committee of Faculty of Pharmacy, girls, Al-Azhar University (REC number: 253). A written informed consent was taken from the patients prior to their enrollment in this study. Patients with other types of cancer, kidney, heart and liver diseases that may affect the parameters were excluded.
2.2. Samples Collection and Methods
- Blood samples were collected according to the recommendations of National Committee of Clinical Laboratory Standards (NCCLS) [16]. Seven ml of venous blood were collected after an overnight fasting by vein puncture from each patient. Each blood sample was divided into two portions:The first portion was used for complete blood picture: two ml of blood samples were collected from each patient into tri-potassium ethylenediamine tetra -acetic acid (K3 EDTA) anticoagulant tubes, and analyzed for CBC in SYSMEX-1800R.The second portion of blood samples (five ml) were collected in plain vacutainers, left to coagulate adequately before centrifugation at 3000 rpm for 10 minutes followed by serum separation. Serum obtained was divided into aliquots and used for estimation of CSA level using fully automated clinical chemistry analyzer, serum creatinine, blood urea nitrogen, uric acid, serum albumin, ALT & AST, serum bilirubin, ALP, LDH, sodium, potassium, calcium, magnesium levels by VITROS 5600/XT 7600 Integrated Systems. Another serum portion was stored at -40°C until time of KIM-1 assessment by ELISA.
2.3. Estimation of cyclosporine A level
- The CEDIA® Cyclosporine PLUS assay was used for the in vitro quantitative determination of cyclosporine A (US, No. 4708929) in serum using automated clinical chemistry analyzer according to the method of Andrews and Cramb [17].
2.4. Estimation of serum Kidney Injury Molecule-1 (KIM -1)
- KIM-1 was estimated by ELISA. The kit used for KIM-1 assay in serum samples was obtained from Bioassay technology laboratory (China, No. E10099Hu) according to the method of Freeman et al. [18].
2.5. Statistical Data Analysis
- IBM SPSS statistics (V. 25.0, IBM Corp., USA, 2017-2018) was used for data analysis. Date were expressed as median and percentiles (1st quartile- 3rd quartile) for quantitative non-parametric data. Comparison between two independent groups was done using Wilcoxon Rank Sum test. Wilcoxon signed rank test was used for comparison between two dependent groups. Comparison between more than 2 patient groups was done using Kruskal Wallis test. Ranked Spearman correlation test to study the possible association between two variables among each group.The probability of error at 0.05 or less was considered significant, while at 0.01 or less was highly significant.
3. Results
|
|
|
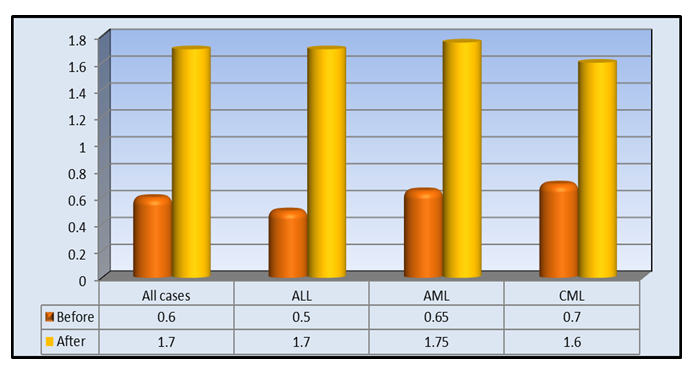 | Figure 1. Median of creatinine (mg/dL) in all studied patients and for each subgroup |
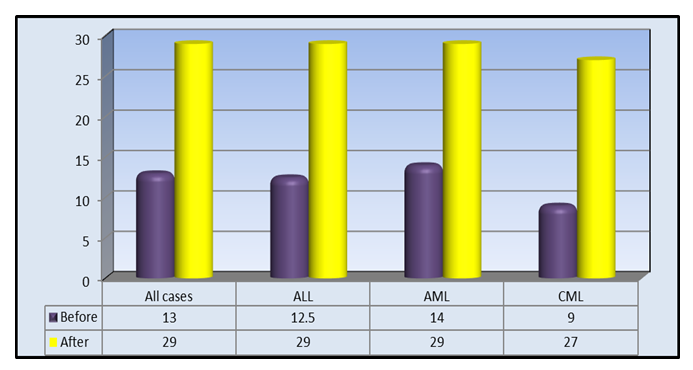 | Figure 2. Median of BUN (mg/dL) in all studied patients and for each subgroup |
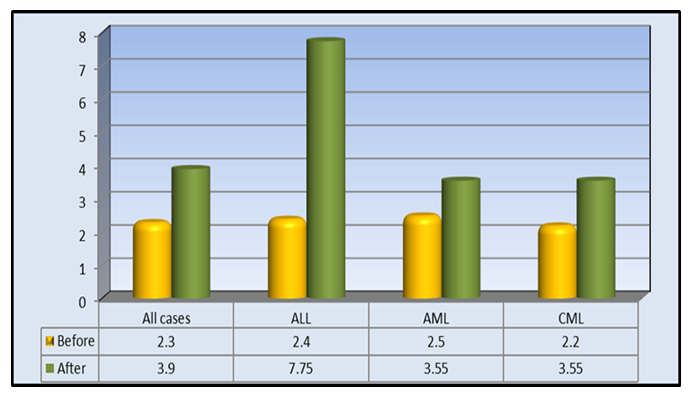 | Figure 3. Median of KIM-1(ng/mL) in all studied patients and for each subgroup |
4. Discussion
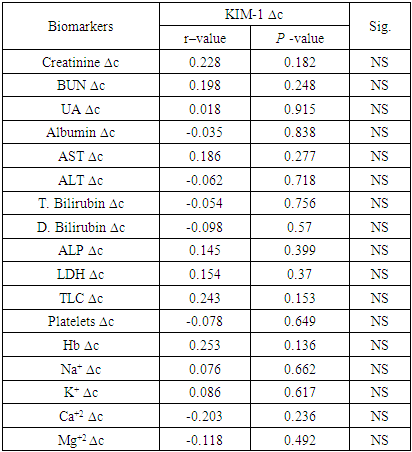
- Nephrotoxicity is considered one of the major side effects of CSA treatment as immunosuppressant following transplantation operations [19]. KIM-1 is a transmembrane glycoprotein that has been demonstrated as an early indicator of kidney injury in rodents and humans [20].In the present study, a comparison between kidney markers before and after CSA treatment revealed highly significant elevations in the levels of creatinine, BUN, uric acid and KIM-1 after cyclosporine treatment than before (Table 1, Figures 1, 2, 3). The kidney affection by CSA treatment was explained by Hoskova et al. who stated that the activation of the renin-angiotensin system and the sympathetic nervous system, increases the production of endothelin-1, and causes induction of oxidative stress and alteration of the NO system. Together these changes cause systemic and renal vasoconstriction leading to the development of endothelial dysfunction, hypertension and kidney damage. They added that the acute effects of CSA nephrotoxicity can even occur after the first dose of CSA is given [21]. Another mechanism by which CSA use leads to renal damage was explained by Krishnappa et al. who stated that CSA decreases renal Klotho expression and increases oxidative stress causing renal endothelial damage. Moreover, they stated that CSA augments the production of renin and vascular endothelial growth factor (VEGF) in renal collecting ducts that causes renal ischemia and periductal fibrosis resulting in nephrotoxicity [22].Fu et al. found KIM-1 significantly increased at all stages of CSA-induced nephrotoxicity, suggesting that CSA-induced tubular injury occurs early, preceding interstitial fibrosis, and is a continuous process during the period of drug exposure [2]. Gohda et al., explained the appearance of KIM‐1 in the circulation following tubular damage by the lack of tubular cell polarity and the increased trans-epithelial permeability because altered microvascular permeability is an important participant in the pathophysiology of kidney injury. The actin cytoskeleton architecture is disrupted in renal microvascular endothelial cells, with loss of cell- matrix and cell- cell adhesion junctions, also endothelial cells are detached from the basement membrane facilitating KIM-1 movement into the circulation [13], [23]. Lu et al. and Song et al. stated that KIM-1 could be considered as an efficient novel biomarker in the diagnosis of AKI within 24 hours after kidney injury, especially in ischemic acute tubular necrosis (ATN) [24], [25]. After a series of researches, KIM-1 was considered as sensitive and specific marker for injury as well as a predictor of the outcomes in humans [26].It’s well known that CSA is hazardous to liver and kidney tissues and can cause massive changes in blood cells count & levels of minerals [27]. So, close monitoring of these markers is important to keep an eye on their levels, reduce CSA dose or even replace it with another immunosuppressive agent in case of alarming results [28], [29].The present study showed highly significant elevations in AST, ALT, total bilirubin, direct bilirubin, ALP and LDH while albumin showed highly significant decline after CSA treatment when compared to before CSA treatment (Table 1).These results were in accordance with the findings reported by Korolczuk et al. who stated that CSA can induce liver injury. In CSA-induced liver injury, morphological and functional changes are observed including elevated serum levels of transaminases and alkaline phosphatase, hyperbilirubinemia, cholestasis, increased bile salts production, impaired trabecular structure, hepatic sinus congestion and widening, activation of the Kupffer cells, passive congestion and oedema of portal tracts, mild mononuclear cell infiltrations within portal tracts, and degenerative changes in the hepatocytes with focal necrosis [27]. They stated also that the development of hyper-metabolic state in the liver and inhibition of ATP-dependent transport of bilirubin and bile salts through the hepatocyte canalicular membranes as well as of bile secretion could explain CSA induced liver impact [27].Moreover, Akbulut et al. suggested the involvement of oxidative stress as one of the mechanisms of hepatotoxicity [30]. Hence, Damiano et al. suggested the use of specific antioxidants of new generation, like Apocynin, to reduce the CSA nephrotoxicity and hepatotoxicity [31].In the present study, hemological parameters (TLC, Platelets and Hemoglobin) showed highly significant decline after CSA treatment when compared to before CSA treatment (Table 1).These results were in accordance with the findings reported by Raeisi et al. and Wu et al. who stated that CSA is implicated in the pathogenesis of thrombotic micro-angiopathy (TMA), a condition results in thrombosis in arterioles and capillaries, due to an endothelial injury, which can be seen in association with thrombocytopenia, anemia and renal failure. TMA is primarily characterized by thrombocytopenia due to platelet aggregation, fragmentation of erythrocytes, and renal injury [19], [32].In the present study, Na+. and K+. showed highly significant elevations while Ca+2. and Mg+2 showed highly significant decline after CSA treatment compared to before CSA treatment (Table 1).These results were in accordance with the findings reported by De Waele et al. who stated that CSA is associated with electrolyte disturbances as CSA increases the fractional excretion of sodium. In addition, hyperkalemia most probably occurs secondary to a direct effect of CSA on the distal renal tubular function and extra-renal potassium handling. Moreover, CSA-induced tubular dysfunction may lead to calcium wasting and distal tubular acidosis [29].In the present study, creatinine, BUN and KIM-1 were significantly elevated in all types of acute and chronic leukemia reflecting a resemblance in kidney status of all recruited patients (figures 1, 2 and 3). Hence, there was no need to deal with different types of leukemia in the present study separately. However, KIM-1 showed the highest elevation in ALL group after CSA treatment (figure 4). This may be attributed to leukemic infiltration of kidney as noted by Bhatia et al. who stated that although leukemic infiltration of kidney may present in all leukemia’s types, it is more often found with lymphoblastic leukemia leading to significant impairment of renal function especially if it is bilateral, diffuse and involves the cortical region. They added that renal complications in acute leukemia can occur due to several factors including leukemic infiltration of the kidneys, therapy or dose-related side effects as tumor lysis syndrome, drugs, and septicemias [33]. Also, Luciano and Brewster stated that acute and chronic leukemias were associated with nephrotic syndrome and glomerulonephritis, leading to the increase of KIM-1 level with all types of leukemia [34]. Whereas, serum creatinine, although its significant increase in all types of leukemia, is a non-specific marker, so that age, gender, muscle mass, and nutritional status all affect serum creatinine levels. Additionally, as a non-discriminating marker of glomerular filtration, serum creatinine can give false results with volume depletion, non-steroidal anti-inflammatory drugs use, or many other causes of decreased renal perfusion without real kidney injury. Even in healthy subjects, serum creatinine shows dramatic alterations with repeated measures [9].Regarding BUN, Rasool et al. reported an increase in BUN in different types of leukemia and reported that patients who had higher levels of BUN, whatever the type of leukemia is, and severe neutropenia, had high mortality rates [35]. However, BUN could not be used as a sensitive marker for kidney injury as changes in serum BUN concentrations primarily reflect functional changes in filtration capacity and is not a true injury marker as it is affected by many factors including protein intake, endogenous protein catabolism, hydration state, hepatic urea synthesis, and renal urea excretion [36].Moreover, the sensitivity and specificity of serum BUN and creatinine are not satisfying. Some primary glomerular diseases and renal tubular diseases may not present with increasing serum creatinine at their early stages while KIM-1 was found as a sensitive and specific biomarker for acute tubular injury (primary or secondary to glomerular diseases) when proximal tubules fail to be repaired as the persistent presence of KIM-1 is involved in the processes of interstitial fibrosis. Hence, it could be used as a predictor of the capacity of renal functional recovery [37,38].Rasool et al. stated that KIM-1 is expressed and excreted in urine within 12 hours after the initial kidney ischemic insult and before regeneration of the epithelium, persisting over time thereafter. They reported it as a noninvasive, sensitive, rapid, and reproducible biomarker of experimental nephrotoxic and ischemic acute kidney injury [35]. Also, Wasung et al. stated that KIM-1 is an excellent biomarker in urine and plasma for the early prediction of AKI [39].In the present study, after follow up of patients for 32 days, the incidence of AKI amongst patients receiving allogeneic stem cell transplantation, in spite of the close monitoring of kidney condition, was 33.3%.Theses finding were similar to Ataei et al., (2015) who reported an incidence of AKI in allogeneic transplant recipients having AML and ALL of 33% while using a conditioning regimen composed of cyclophosphamide and busulfan [40]. Krishnappa et al. stated that CSA nephrotoxicity is the main factor of kidney failure, especially during 20–33 days after SCT when CSA levels in blood starts declining [22]. So, after 32 days, the data of the patients were further divided into 2 groups according to nephrotoxicity status and the comparison between kidney markers revealed that KIM-1 was superior to other kidney markers including creatinine, BUN and urea in predicting the risk of nephrotoxicity as the levels of KIM-1 showed significant differences between positive and negative nephrotoxicity groups regarding before, after CSA and ∆Change values (p≤0.01, p≤0.01, p≤0.02) respectively, while the only significant difference obtained regarding creatinine was after CSA treatment (p≤0.02). Moreover, Urea & BUN showed no significant differences between positive and negative nephrotoxicity groups (Table 2).Correlation coefficient between ∆Change of KIM-1 and ∆Change of other studied biomarkers in the present work revealed no significant difference between ∆Change of KIM-1 and any of the studied parameters even the traditional kidney markers (Table 3). These results support our hypothesis that KIM-1 elevation is not dependent on the same mechanisms of other kidney markers. Kellum et al. stated that creatinine assesses renal function loss rather than kidney tissue injury. Whereas, KIM-1 is specific for kidney injury when the proximal tubules fail to be repaired [8], [38].Also, in a study by Rouse et al. KIM-1 when compared to other kidney markers as BUN, creatinine, osteopontin and clusterin exhibited the earliest and the largest response to gentamycin-induced AKI. Moreover, it also correlated with the evolving histopathologic changes and outperformed serum creatinine. During recovery, KIM-1 reflected tubular regeneration precisely [41].
5. Conclusions
- KIM-1 is a useful biomarker for the early prediction of kidney affection caused by CSA treatment in leukemic patients performing stem cells transplantation. Serum KIM-1 was superior to other traditional kidney markers in monitoring kidney condition so could be used with critically ill individuals as leukemia patients or those under rigorous treatment as CSA for close watch of kidney status.
ACKNOWLEDGEMENTS
- We would like to thank all staff members of hematology, Bone Marrow Transplantation departments and technicians of chemistry laboratory department in Nasser Institute Hospital for their great help during this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML