-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Tumor Therapy
p-ISSN: 2163-2189 e-ISSN: 2163-2197
2019; 8(1): 10-15
doi:10.5923/j.ijtt.20190801.03

Effect of a Novel Dietary Supplement on Cancer Cells Metabolism
Antonelli Francesco1, Ferorelli Pasquale1, De Martino Angelo1, Borromeo Ilaria1, Shevchenko Anna2, Beninati Simone1
1Department of Biology, University of Tor Vergata, Rome, Italy
2Department of Pharmacology, Kabardine University, Russia
Correspondence to: Beninati Simone, Department of Biology, University of Tor Vergata, Rome, Italy.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

One area of research currently garnering significant attention is the in-depth study of cancer cell metabolism. The purpose of this research is not exclusively to discover new anticancer therapies, but rather to improve the quality of life of the patient. Many cancer patients suffer from a muscle wasting syndrome called cachexia. Cancer related cachexia impairs quality of life and response to therapy, which increases morbidity and mortality among cancer patients. The data presented in this report were collected to address disorders caused by therapies aimed at reducing tumor growth. Carbohydrate metabolism is the major pathway in the cell providing energy and building blocks for macromolecule biosynthesis. Glucose metabolism in cancerous cells is remarkably different from that in their normal counterparts. The purpose of this research was to investigate the effects on energy metabolism in normal and neoplastic cells of the various components of the dietary supplement Texidrofolico (TXF), which act on their cellular enzymatic complexes. Treatment of HepG2 tumor cells with TXF reduces glucose consumption, and slows reduction of pyruvate to lactate, following indirect inhibition of lactate dehydrogenase (LDH) by blocking regeneration of NAD+ from NADH. These activities inhibit glycolysis, with consequent reduction in energy yield, evidenced by an increased ADP/ATP ratio. By contrast, in normal MEF cells treated with TXF, the ADP/ATP ratio was observed to be lower than in untreated control cells, showing increased energy relative to untreated normal cells. The observed effects seem to be due to the presence of two active components in TXF: folic acid, and pyruvate. Calciferol, which exerts less influence on glucose consumption, instead shows some inhibition of LDH reductase activity.
Keywords: Dietary supplements, HepG2 cell, MEF cell, Folic acid, Pyruvate, Calciferol
Cite this paper: Antonelli Francesco, Ferorelli Pasquale, De Martino Angelo, Borromeo Ilaria, Shevchenko Anna, Beninati Simone, Effect of a Novel Dietary Supplement on Cancer Cells Metabolism, International Journal of Tumor Therapy, Vol. 8 No. 1, 2019, pp. 10-15. doi: 10.5923/j.ijtt.20190801.03.
Article Outline
1. Introduction
- The quality of life of people with cancer is still neglected. Clinicians rarely face this and few patients talk to their doctor. In most studies, quality of life is excluded from the criteria for assessing the effectiveness of a treatment. Exhaustion, nervousness, difficulty falling asleep, mild dysentery, lack of appetite and cachexia, are serious and very frequent annoyances among cancer patients, which can worsen the quality of life. In Italy currently, almost 4 million patients live after a cancer diagnosis with disorders that, underestimated may compromise adherence to treatments and the effectiveness of treatments. It is important to evaluate the factors involved in the non-compliance with the indicated therapy, including the appearance of side effects. Currently, in several neoplasms, survival increases significantly and the side effects decrease and change. Suffice it to say that survival after five years reaches 91% in prostate cancer and 87% in breast cancer. This new reality requires the need to investigate, analyze and understand the new needs of patients, so that they can improve their quality of life. In particular, the conditions must be set so that on the one hand the patient, his family members and caregivers express the new needs. The project “Search for new dietary supplements to improve the quality of life of cancer patients”, began in 2016, in the laboratory of Experimental Oncology of the University of Tor Vergata, Dept. of Biology, with the realization of four surveys conducted to take a photo on awareness on the subject involved (patients, oncologists, family doctors and pharmacists). The oncologist's goal is not only to increase survival, but also to seek the clinical benefit for the patient who combines stability of disease and quality of life that is good or at least acceptable. If the therapies prolong the time in which the disease is controlled / stable we could say that the disease becomes chronic and in truth (at least for some tumor diseases) more and more sick people cohabit for years with the tumor, more or less symptomatic, continuing to play a social life and relationships, often not very different from the one they had before the pathology. In conclusion, the impact on a patient's quality of life must be a fundamental parameter for establishing the value of a new anti-cancer drug. The determination of clinical efficacy is no longer enough. This is an inevitable process in relation to the provisions of the European Health Technology Assessment (HTA) proposal, presented in January 2018 in Brussels. In order to contribute to the achievement of a protocol aimed at improving the quality of life of the cancer patient, our research group has investigated a series of elements contained in a commercial food supplement, called “Texidrofolico” (TXF), highlighting the potential influence of these on the cellular metabolism. A subsequent study will focus on the administration of this supplement to cancer patients, in order to be able to observe any positive effects on some of the most common disorders due to the pathology in place and to chemotherapy (work in progress).Long-term investigation and extensive efforts using genomics, proteomics or metabolomics analysis identified thousands of mutations in a single tumor. However, we cannot succeed at curing cancer by targeting mutations as the cause of cancer. Therefore, as an alternate therapeutic approach from classical oncology study, stimulation of the inherent ability of the immune system to attack tumor cells was welcome as a new principle in cancer therapy. Targeting the cancer energy metabolism may be a cancer-specific therapy for all kinds of cancer because normal cells do not rely on cancer energy metabolism under normal conditions.Enzymes are one of the main components of the human organism, they are catalysts of biological processes and are, therefore, responsible for vital processes in both animals and plants [1]. Changes in cell function are achieved by altering the activity of these small protein agents, either through changes in gene expression, protein degradation or post-translational modifications [2]. The numerous molecular processes in cells yield complex cellular structures, movements and regulatory decisions. We know most of the molecules that underlie these structures, behaviors and decisions, but we often do not know how all these molecules interact and function together [3]. The purpose of this research was to investigate the role of the various components of a food supplement (TXF) in intervening in the energy metabolism of normal and neoplastic cells, by acting on the cellular enzymatic complex, to reduce the side effects of anticancer therapies, improving the quality of life of cancer patients.
2. Experimental Protocol
2.1. Cell Treatments
- Two cell lines were used as the in vitro model. A primary culture of human fibrocytes (Immortalized Mouse Embryonic Fibroblast - MEF) and a tumor cell line of human hepatocarcinoma (HepG2). On the basis of some previous experiments, were also evaluated some particularly active components of the formulation of TXF, such as: calciferol (vitamin D), folic acid and pyruvic acid. TXF was obtained from CITOZEATEC, S.r.l. (Peschiera Borromeo, Milano, Italy). The main components of TXF are as follows (units/100 g): 500 mg of vitamin C, 56 mg of vitamin B5, 56 μg of vitamin D, 3.3 mg of vitamin B9, 3.3 mg folic acid, 222 mg of pyruvic acid, 120 mg of citric acid and 250 mg of tartaric acid, amylase and lactase.
2.2. Dosage of Glucose Consumption
- Glucose consumption was evaluated in both cell lines in 6-well plates. Glucose consumption was determined using a glucose-oxidase test kit (GAGO-20, Sigma Aldrich, MO, USA). After the TXF treatment (2 mg/mL), at different times (3, 6, 12, 24 and 48 hours), 50 μl of culture medium was collected from untreated and treated groups.
2.3. [ADP] / [ATP] Ratio Measurement
- The ADP/ATP ratio, when measured properly, provides a snapshot into the bio-energetic status of a cell or tissue. It has been successfully used to assess the metabolic state of a variety of tissues under various conditions [4]. The cells were grown in 96-well plates with a transparent bottom. The [ADP] / [ATP] ratio of both cell lines was determined immediately after the incubation period (3, 6, 12, 24 and 48 hours) using an assay kit (MAK135; Sigma Aldrich, USA) according to the manufacturer's instructions. Luminescence levels of the luciferase-mediated reaction were measured using a plate illuminometer (PerkinElmer 2030 multi-reader, MA, USA). The ratio [ADP] / [ATP] was calculated from the luminescence values using a formula provided by the kit manufacturer.
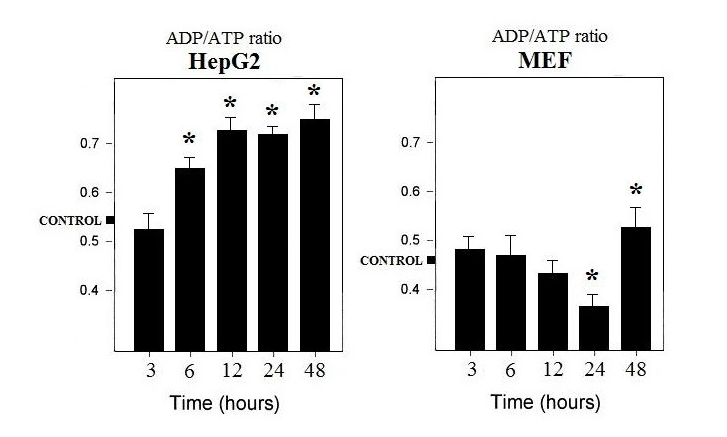 | Figure 2. ADP/ATP ratio in fully formulated TXF-treated HepG2 and MEF cells. The value represents the mean of three determinations with ± SD, *p<0.05 |
2.4. Assay of Lactate Content
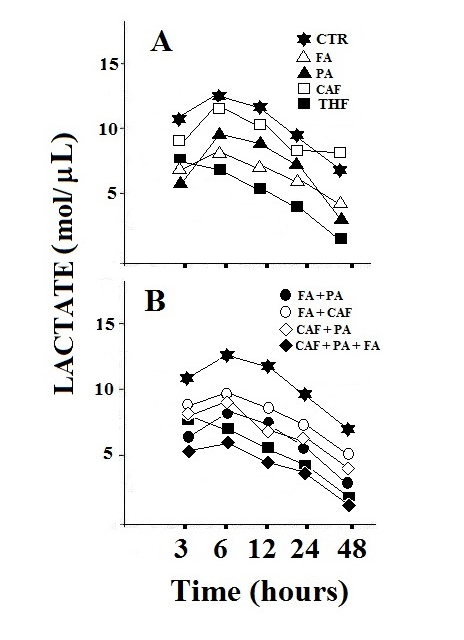
- The concentration of lactate, the final product of anaerobic glycolysis, was determined using a lactate colorimetric assay kit (Abcam, MA, USA). The cells were grown in 6-well plates for 3, 6, 12, 24 and 48 hours with and without TXF (2 mg/mL). The cells were collected and then harvested and deproteinized according to the manufacturer's protocol. The optical density was measured at λ450 nm and a standard curve graph (nmol/well vs. OD λ450 nm) was generated using serial dilutions of lactate. Lactate concentrations were calculated using the formula provided by the kit manufacturer.
2.5. Cell Cycle Assay and Apoptosis
- Cells were grown in 6-well plates and treated with 2 mg/mL of TXF or 0.5 mg/mL of calciferol, folic acid and pyruvic acid as individual components or in various combinations of them. After culture for 3, 6, 12, 24 and 48 hours, the cells were collected and subjected to cell cycle analysis by staining with propidium iodide as previously reported [5]. Data were obtained by flow cytometry using Accrui (BD Biosciences, NJ, USA) and analyzed using FlowJo software (Tree Star Inc, OR, USA).
2.6. LDH Assay
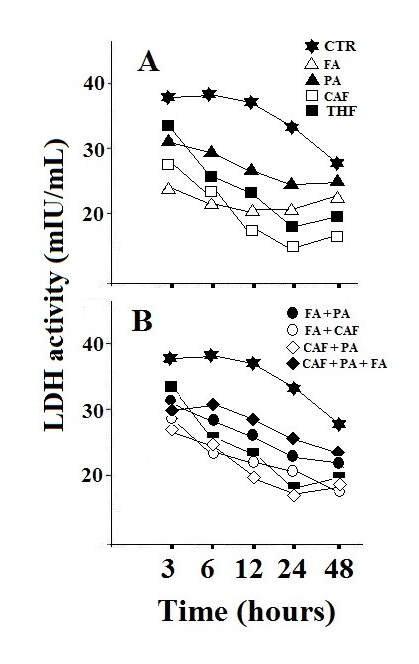
- The enzyme activity of LDH was evaluated with the Vanderlinde method [6]. In brief, HepG2 hepatocytes and MEF cell were cultured in 96-well plates. After treatment for 3, 6, 12, 24 and 48 hours in DMEM supplemented with and without TXF (2 mg/mL), LDH was measured in medium and cell extracts according to the manufacturer’s instruction.
2.7. Statistical Analysis
- All the above tests were performed in triplicate and repeated at least three times. Statistical significance was defined as p-value below 0.05 and determined by t-test and two-way ANOVA test for the rest of the GraphPad Prism tests (v7.03, Graphpad Software Inc., CA, USA); error bars indicated standard deviation.
3. Results and Discussion
- Several pathways affected by TXF, have been validated, and used as a focal point for further investigation, to identify specific molecular targets mediating the enzymatic metabolism of normal and cancer cells.
3.1. Suppression of Energy Metabolism by TXF on Cancer Cells
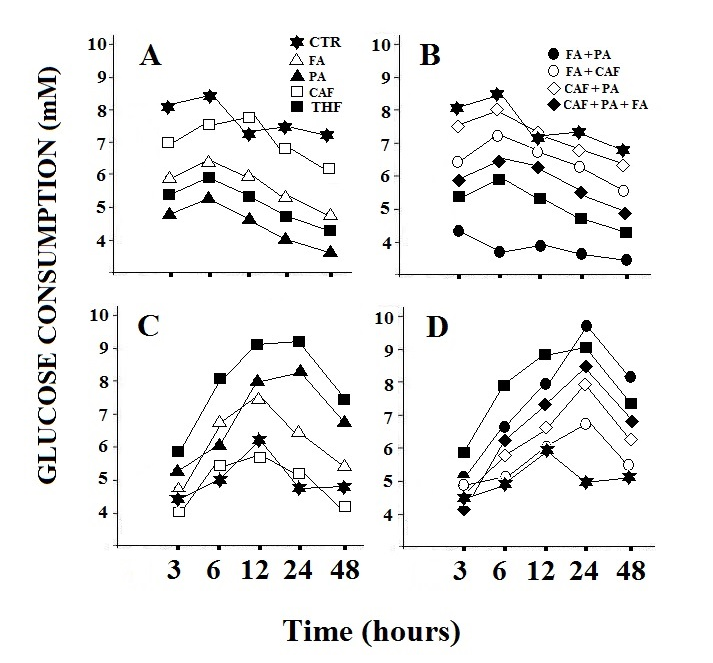
- A preliminary screening was carried out to highlight the potential active components of TXF (data not shown). The results obtained allowed us to identify three components of TXF, particularly active on cellular metabolism: folic acid, pyruvate and calciferol.From Figure 1 it is possible to observe that the treatment of HepG2 cells with TXF reduces the consumption of glucose, with consequent inhibition of the energy metabolism of the cancer cell. In fact, the obtained data, seem to suggest that HepG2 cells, in the presence of pyruvic acid and folic acid, two main components of TXF, use lower amounts of glucose than the control (Figure 1A and B), reducing energy yield as confirmed by a higher ratio [ADP] / [ATP] (Figure 2). On the contrary, the TXF-treated MEF cells significantly increased the glucose consumption (over 55%) likely due to the presence of pyruvic acid and folic acid in the TXF formulation (Figure 1C and D). Furthermore, in normal MEF cells, the [ADP] / [ATP] ratio is lower, thus showing an increase in energy metabolism (Figure 2).Intracellular ADP and ATP levels fluctuate rapidly because these high-energy phosphate molecules are rapidly produced and consumed in many intracellular biochemical reactions. It is widely known that viable cells turn over entire ATP stores on the order of minutes, and that dying cells are incapable of replenishing their ATP stores when depleted [7]. The 40% reduction in glucose consumption and higher lactate levels (Figure 4), observed in cancer cells suggests the inhibition of the Krebs cycle or oxidative phosphorylation. In this context among the various components of TXF, folic acid and pyruvate, appeared the most active factors (Figure 1A and B). In contrast, calciferol did not show a significant effect on glucose consumption. Again, these observations, in combination with the other results, supports a decrease in metabolic flow through the Krebs cycle as the likely cause of suppression of the tumor cell’s energy metabolism.
3.2. Perturbation of the Cell Cycle by Treatment with TXF
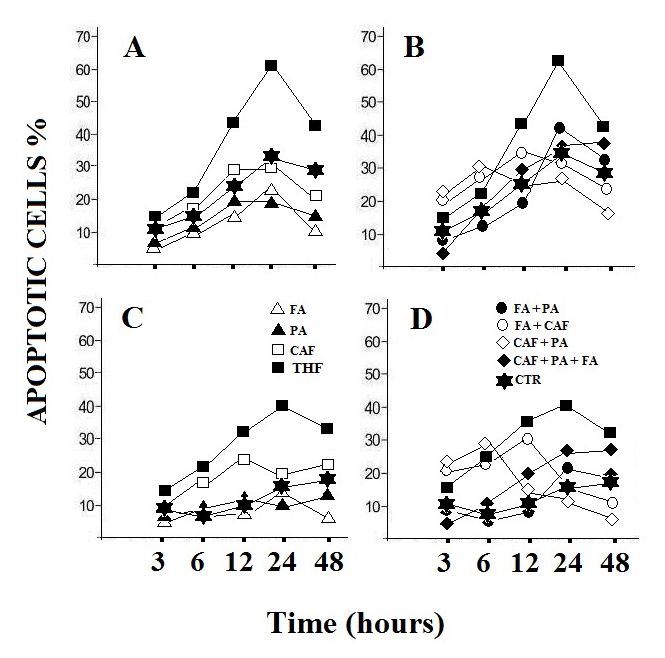
- Figure 3 shows the induction of apoptosis in the HepG2 cell line by the three active components alone (Figure 3a) or in combination (Figure 3b) with respect to TXF in its complete formulation. From the results it can be deduced that TXF can influence the cell cycle, by altering the proportions of apoptotic cells in tumor cell cultures treated at different times compared to controls (Figure 3 a). Folic acid, the most abundant compound present in TXF, has been previously shown to disrupt a number of signaling pathways and alter/stop the cell cycle in a variety of cancer cells [10]. This observation was confirmed in HepG2 cells. The present results allow a direct comparison with TXF because our experiments were conducted using equivalent concentrations of folic acid, calciferol and pyruvic acid similar of those present in TXF. Folic acid, calciferol and pyruvic acid do not appear to be as effective in influencing the cell cycle as the complete product. This indicates that these three components should interact with other compounds present in TXF to have a greater effect on the cell cycle. The normal cell line (MEF) is not affected by TXF treatment or by the three components at different times (Figure 3 c, d). In conclusion, it is possible to deduce that TXF-treated HepG2 cells drastically reduce the consumption of glucose which lowers the available ATP levels. This condition is sufficient to trigger the programmed cell death of tumor cells [11], as can be deduced from Figure 3.
3.3. Metabolic Effects of TXF on Normal Cells
- In an ongoing investigation, in patients with lung cancer, we observed that anorexia, weakness, cough, dyspnea, hemoptysis and pain, typical symptoms of chemotherapy, are reduced in the combined treatment with Pemetrexed-TXF compared to that with Pemetrexed alone (work in progress). On the basis of the observations presented in this report, it is feasible to understand how the complete formulation of TXF can act positively on the symptoms typical of cancer patients undergoing chemotherapy. It is interesting to note that in normal MEF cells, TXF has the opposite effect on the ratio [ADP] / [ATP] compared to that observed on cancer cells, indicating that the complete formulation of this supplement, in normal cells, can increase the energy metabolism and increase the energy load of the cell. This is particularly interesting considering the observations made on cancer patients treated with TXF. In these patients not only was the decrease in the negative effects of cancer cachexia observed, but also an increase in vital signs with a consequent better quality of life (work in progress).
4. Conclusions
- The experimental observations, although preliminary, would suggest that the burst of additional energy deriving from the treatment with TXF, increases the performance of normal cells in the body, improving the quality of life of the patient. On the contrary, the reduction of energy generated by TXF in cancer cells may slows down the disease progression. These findings may explain the positive effects observed in patients with lung cancer. Furthermore, it is not difficult to suppose that treatment with TXF may be involved in activating the patient's immune system due to the presence of additional glucose, which is not used by cancer cells. Obviously all these assertions will have to be investigated in the near future. Therefore, in conclusion, we could deduce that the food supplement tested could be an excellent adjunct in the treatment of cancer patients, obviously not as an antitumor drug, but as useful to improve the quality of life by reducing the adverse symptoms of chemotherapy.
ACKNOWLEDGEMENTS
- We are grateful to A.R.S.S. Italy, for financial support for studying the possibility of improving the quality of life of cancer patients.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML