-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Tumor Therapy
p-ISSN: 2163-2189 e-ISSN: 2163-2197
2019; 8(1): 1-3
doi:10.5923/j.ijtt.20190801.01

Immune Checkpoint Blockade Therapy for Cancer
Nader Hazboun
Biology Department, Bethlehem University, Bethlehem, Palestine
Correspondence to: Nader Hazboun, Biology Department, Bethlehem University, Bethlehem, Palestine.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The expression of immune checkpoint molecules such as programmed cell death ligand 1 (PD-L1) on the surface of cancer cells and programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) on the surface of T-cells results in immune tolerance. Despite the unprecedented success of checkpoint inhibitors in cancer therapy, only a minority of patients respond durably. This review focuses on the role of immune checkpoint blockade in cancer therapy highlighting clinical trials with the best results, mechanisms of resistance, combination therapies and future directions in this interesting field.
Keywords: Immunotherapy, Immune checkpoint molecules, Monoclonal antibodies
Cite this paper: Nader Hazboun, Immune Checkpoint Blockade Therapy for Cancer, International Journal of Tumor Therapy, Vol. 8 No. 1, 2019, pp. 1-3. doi: 10.5923/j.ijtt.20190801.01.
Article Outline
1. Introduction
- Immune checkpoint molecules enable self-tolerance under normal physiological contexts but frequently become coopted in malignancy [1]. The treatment of cancer with immune checkpoint inhibitors targeting cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and the programmed cell death 1 and programmed cell death ligand 1 (PD-1/PD-L1) axis have shown unprecedented clinical activity in several cancer types [2]. Only a subset of patients respond, but those who achieve a response have long-term disease control. One of the main reasons for the low response rate and relapse is primary (de novo) and acquired resistance to immune checkpoint inhibitors, respectively. The increased understanding of the biology of current and novel checkpoint molecules enables the rationale design of combination immunotherapy strategies so that a large number of patients with different tumor types will respond durably. This review summarizes the basic biology and clinical aspects of immune checkpoint blockade therapy for cancer.
2. CTLA-4 and PD-1/PD-L1 Immune Checkpoints
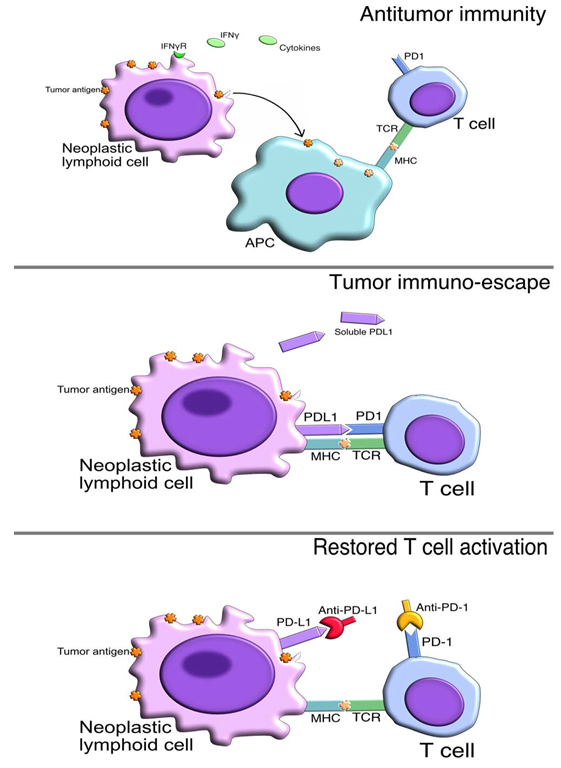
- In order to become fully activated, T-cells must encounter antigen in the context of antigen-presenting cells (APCs) such as dendritic cells, which provide co-stimulatory signals mediated by B7 molecules (B7-1 and B7-2) that will engage their ligand, CD28, in the T-cell [3]. A pivotal moment occurred when a protein known as cytotoxic T lymphocyte-associated protein 4 (CTLA-4) was demonstrated to have a potent inhibitory role in regulating T cell responses [4]. In resting T cells, CTLA-4 is an intracellular protein; however, after T cell receptor (TCR) engagement and a co-stimulatory signal through CD28, CTLA-4 translocates to the cell surface, where it outcompetes CD28 for binding to critical co-stimulatory molecules (CD80, CD86) and mediates inhibitory signaling into the T cell, resulting in arrest of both proliferation and activation [4]. The PD-1/PD-L1 axis is one of the major mechanisms of immune escape exerted by several cancer types in which up-regulation of PD-L1 is observed. PD-L1 interacts with its inhibitory receptor PD-1 expressed on activated T cells with the function of promoting self-antigen tolerance and balances the immune regulation to avoid antigen persistence and immune-mediated pathologies (Fig.1) [5].
3. Clinical Trials of Checkpoint Blockade
- The use of checkpoint inhibitors that either block CTLA-4 such as ipilimumab or block the PD-1/PD-L1 axis such as nivolumab (anti-PD1), pembrolizumab (anti-PD1) or atezolizumab (anti-PDL1) have led to a paradigm shift in the treatment of patients with solid tumors. The following section summarizes the best results of clinical trials with immune checkpoint inhibitors (ICI) in different tumor types.
3.1. Checkpoint Blockade in Lung Cancer
- In a phase III trial of nivolumab vs docetaxel in 272 patients with squamous non-small cell lung cancer (NSCLC), the overall response rate was 20% in the nivolumab arm with a median overall survival of 9.2 months [6]. Seven percent of patients treated with nivolumab had grade 3/4 adverse events [6].
3.2. Checkpoint Blockade in Colorectal Cancer
- Currently, there are many actively recruiting clinical trials with either anti-PD-1 or anti-PD-L1 mAbs for treatment of metastatic colorectal cancer (mCRC) as first or 2nd/3rd line treatment in combination with other checkpoint inhibitors or standard therapy [7]. The checkmate-142 study (NCT02060188) is a phase 2 study of nivolumab and combinations of nivolumab and other inhibitors in mCRC and its interim results are encouraging for the combination therapy in microsatellite instability (MSI) patients [8]. Phase I/II trials of PD-1/PD-L1 targeted therapy showed grade 3-4 immune-related adverse events (IRAE) such as endocrinopathies, hepatitis, and colitis in up to 41% of patients [7].
3.3. Checkpoint Blockade in Hepatocellular Carcinoma
- A phase II clinical trial blocking CTLA-4 with a monoclonal antibody has been carried out in 21 patients with un-resectable hepatocellular carcinoma (HCC) of Child-Pugh class A or B (Clinicaltrials.gov number NCT01008358) [9]. Each patient received at least two treatment cycles of 90 days. Seventeen patients were evaluated for tumor response; three patients had a partial response that lasted for up to 15.8 months, and stable disease was observed in further 10 patients with half stabilized for greater than 6 months [9].
3.4. Checkpoint Blockade in Melanoma
- In a study that combined nivolumab with ipilimumab (anti-CTLA4) in untreated melanoma patients, the overall response rate (ORR) was 57.6% as compared with 19% in the anti-CTLA4 monotherapy group but with higher adverse events in the combination group [10].
3.5. Checkpoint Blockade in Lymphoma
- In a phase I trial of pidilizumab (anti-PD1) in 66 patients with diffuse large B-cell lymphoma (DLBCL), the ORR was 51% with 96% adverse events, while in a phase I trial of nivolumab in 23 patients with Hodgkin’s lymphoma, the ORR was 87% with 22% adverse events of grade 3 only [11].
3.6. Checkpoint Blockade in Multiple Myeloma
- A phase Ib study of PD-1 blockade with nivolumab was recently completed in 27 patients with relapsed or refractory multiple myeloma [12]. Stabilization of disease was observed in 17 patients (63%), which lasted a median of 11.4 weeks [12].
4. Biomarkers of Response to Checkpoint Blockade
- Tumor cell PD-L1 expression has been postulated as a predictive biomarker of response to immune checkpoint inhibitors, however, response rates remain below 60% even in patients expressing higher levels of PD-L1 [13]. Several other dynamic biomarkers may help direct personalized ICI therapy; these include T-cell infiltrate and functionality, major histocompatibility complex (MHC) expression status, neoantigen burden, metabolic status, and general immune status factors such as lymphocyte count [13].
5. Mechanisms of Resistance to Checkpoint Blockade
- Enumerating the underlying mechanisms of de-novo (or primary) and acquired resistance to immune checkpoint targeting has become a logical next step in cancer research [1]. These mechanisms include defects in tumor immunorecognition such as genetic deficiencies in β2-microglobulin and depletion of the neoantigen repertoire, insensitivity to immune effector molecules such as mutations in the interferon-γ signaling pathway and the caspase 8 gene involved in the extrinsic apoptosis pathway, immunosuppressive tumor microenvironment and neovasculature, tumor plasticity and stemness, the enteric microbiome, and finally cooption of alternative immune checkpoints such as T-cell molecule with immunoglobulin and mucin domain 3 (TIM-3) [1].
6. Combination Therapies with Checkpoint Blockade
- From a mechanistic perspective, it is possible that combination strategies with immune checkpoint therapies and genomically targeted agents such as the BRAF inhibitor vemurafenib (a tyrosine kinase inhibitor) will result in induction of immune memory leading to more durable control of tumor growth than what is achieved with each therapy alone [3]. The targeted agent will induce immunogenic cell death leading to the release of tumor neoantigens which can be presented by antigen-presenting cells (APCs) to tumor-specific T cells, which become activated and upregulate inhibitory checkpoints such as CTLA-4 and PD-1 that could then be blocked with antibodies to permit enhanced anti-tumor T-cell responses [3]. Another combination therapy with ICI involves immunomodulatory drugs such as lenalidomide and pomalidomide; in a phase 1 study that involved 17 patients with relapsed/refractory MM (NCT02036502), the combination of lenalidomide and low-dose dexamethasone with pembrolizumab resulted in a 76% response rate [14]. Other potential combinatorial approaches with ICI involves targeting the immunosuppressive network in the tumor microenvironment such as inhibiting regulatory T-cells, type 2 macrophages, indoleamine 2,3-dioxygenase or arginase [15].
7. Conclusions & Future Perspective
- The expression of inhibitory receptors on T cells, such as PD-1 and CTLA-4, contributes to dysfunctional effector T cell responses. Therefore, therapies that block these inhibitory receptors or their ligands have shown remarkable efficacy in inducing an anti-tumor immune response in a number of tumor types [16]. However, many patients still do not respond to these treatments and those who respond eventually relapse. The combination of checkpoint inhibitors with each other, with chemotherapy or with other immunomodulatory agents would increase the response rate in cancer patients and decrease the risk of relapse due to secondary resistance making checkpoint blockade an important paradigm shift in cancer therapy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML