-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Tumor Therapy
p-ISSN: 2163-2189 e-ISSN: 2163-2197
2018; 7(1): 27-33
doi:10.5923/j.ijtt.20180701.02

Expression of CD44 and CD133 in Glioma Stem Cells
Sima Fakhari1, Ali Zare Mehrjardi1, Mitra Noori2
1Department of Pathology, Iran University of Medical Sciences, Tehran, Iran
2Department of Biology, Faculty of Science, Arak University, Arak, Iran
Correspondence to: Sima Fakhari, Department of Pathology, Iran University of Medical Sciences, Tehran, Iran.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Gliomas are the most frequent primary central nervous system neoplasms. Cancer stem cells have been implicated in tumor initiation, progression, metastatic potential and resistance to therapy. Aim of this study is determination of CD44 and CD133 expression level as cancer stem cell markers in different gliomas comparing to normal brain tissue and its correlation with tumor grade. Study carried out on 31 low grades (I & II), 36 high grade (III & IV) glioma patients and 12 normal brain tissue samples. CD44 and CD133 markers expression levels examined using immune-histochemical staining method. Correlation between two these markers with glioma grade and their expression level were studied in different gliomas and human normal brain tissue. Data were analyzed using SPSS in Bivariate correlation and Simple liner regression tests. The positive expression rates of CD44 (44/67) and CD133 (9/67) in patients with gliomas were higher than those in normal brain tissues (both 0/12). These markers were expressed in cell membrane and cytoplasm. Significant correlation was seen between stained cells percent and glioma grade in CD44 marker (Cs=0.28). Non liner regression of CD44 and CD133 markers expression was observed in the studied gliomas (R=0.28, P=0.23).
Keywords: Glioma, CD133, CD44
Cite this paper: Sima Fakhari, Ali Zare Mehrjardi, Mitra Noori, Expression of CD44 and CD133 in Glioma Stem Cells, International Journal of Tumor Therapy, Vol. 7 No. 1, 2018, pp. 27-33. doi: 10.5923/j.ijtt.20180701.02.
Article Outline
1. Introduction
- Glioma is the most common primary neoplasm in the central nervous system that graded as I–IV according to the World Health Organization (WHO) system based on histological features [1]. In recent years the diagnosis and treatment of gliomas have made great progress; however, the prognosis of patients with high grade glioma remains poor [2]. Recent studies have shown that gliomas may be caused by tumor stem cells, along with the expression of some characteristics of neural stem cells [3]. The studies have suggested that tumor biology and treatment resistance are closely related to cancerous stem cells [4, 5]. Cancer stem cells (CSC) are found in a variety of cancers, including glioblastoma. A variety of markers such as CD133, CD15, A2B5, Nestin, Musashi-1, BMI1, SOX2, IB1, Id1, and OCT4 have been studied as glioblastoma stem cells markers [6]. CD133 is known as a marker of stem cells in normal tissue and cancers. Several studies have shown the use of CD133 in the nutrition of cells with fundamental properties. But there is also a great deal of evidence limiting the use of stem cell markers [7-10]. Recent evidence suggests that the correlation between CD133 expression, GSCs and molecular subtypes are more complex than initially proposed [11]. CD44 is a major receptor of stem cell surface that is created as multiple isoforms. These isoforms are produced by intermittent connections of at least 10 exons and encoding portions of the extracellular domain [12].Determining approved markers for the accurate diagnosis of gliomas in this decade is still controversial [9]. The results of poor recovery of glioblastoma patients suggest that knowledge of markers for diagnosis and progression of disease are vital to effective treatment and the study of CD44 and CD133 markers in patients can be a valuable method in the diagnosis of glioblastoma multiforme in comparison with control. These tumor markers were determined by micro-assay analysis of glioblastoma specimens. The results showed a significant correlation between expressions of five markers, including CD44, with malignant glioma [13]. Anido et al. (2010) showed that the high level of expression of both CD44 and ID1 markers has an inverse correlation with the rate of survival and prognosis in patients with glioblastoma multiforme [14]. Zhang et al. (2008) studied on the association between the expression of Nestin and CD133 markers with glioma grade and their prognostic value using SABC immunohistochemical analysis. Their results showed a dense and dotted stainability, with a significant increase in expression of these two markers by increasing the grade of gliomas. There was a positive correlation between expressions of these two markers in different types of glioma tissues [15]. The cells that express CD133 marker exhibit more proliferation and cells that express the CD44 marker exhibit more invasions. CD133/OLIG2 or CD44 expression can predict response to radiotherapy [16]. The ratio of CD133+ cells as an unrelated risk factor for tumor growth and progression in WHO Grade II and III tumors was described [17]. A limited collection of recent reports illustrates the correlation between CD133 expression and poor prognosis in brain tumors. CD133+ brain tumor cells resistant to chemotherapy and radiotherapy can be evaluated with precise and preclinical studies. Different results have been obtained regarding the role of CD133 and other stem cell markers in their classification [18].The aim of this study was to determine the expression of CD44 and CD133 markers in different types of gliomas in comparison with normal brain tissue and its correlation with tumor grade in patients referred to Firoozgar Hospital, Tehran, during the years 2014 to 2016.
2. Materials and Methods
2.1. Cases
- This study was performed on 67 patients including 25 female patients aged 4 to 71 years old with mean age of 33.83 and 42 males aged 4 to 67 years with a mean age of 41.48 in Neurosurgery Department of Firoozgar Hospital, Tehran, Iran, that had surgically resected gliomas during 2014 to 2016. They had no history of chemotherapy or radiotherapy before surgery. The World Health Organization (WHO) has defined four grades of gliomas, along with increased histological changes in the tumor [7]. Tumors in grades II through IV are diffuse with invasion to normal brain. Grade II tumor are also called low grade gliomas, Grade III tumors are called anaplastic and grade IV tumor are known as glioblastoma multiforme (GBM). In this study, 31 patients were low grade (WHO grade I & II) and 36 patients with high grade (WHO grade III & IV). Twelve normal brain tissues were used as a control sample for immunohistochemical studies.
2.2. Immunohistochemical Staining Method
- The expression of CD44 and CD133 markers in glioma tissues was studied by immunohistochemical staining. Samples were fixed in 10% buffered formalin and embedded in paraffin. Anti-CD44 Antibody (Phagocytic Glycoprotein-1, HCAM) (DF1485) prepared from BiogenexCo., and Human Anti-CD133 Monoclonal Antibody (SB-019772) from Sina Biotech Inc. were used. Immunohistochemical staining was performed using Avidin-biotin and Dako kit (DAKO ABC kit, UK).Paraffin blocks were selected for each patient and four micrometers sections were prepared. Deparaffinized sections were then treated with methanol and 3% hydrogen peroxide for 10 minutes. Antigen retrieval method was using a microwave oven at 95°C for 20 minutes and then cooling at 25°C for 2 hours. After washing with PBS, blank serum was added for 10 minutes. The sections were incubated with monoclonal Anti-CD133 (1:30) and Anti-CD44 antibodies (1: 100 ready to use) and negative control samples were incubated with PBS instead of primary antibody for 2 hours at 4°C. The negative control sections were incubated with PBS instead of the primary antibody. After washing in PBS, a secondary biotin-marked antibody was applied for 10 min followed by peroxidase-marked streptavidin for 10 min. The reaction was visualized using (3,3'-diaminobenzidine tetrahydrochloride and the nuclei were colored by hematoxylin. Negative and positive controls were routinely used. A number of samples were randomly selected and staining repeated.
2.3. Quantification of the Immunostaining in Human Gliomas
- Immunohistochemical staining for CD44 and CD133 markers in tumoral cells was scored. The number of positive staining cells indicating immunoreactivity on cell membrane and cytoplasm was counted in 10 microscopic fields and the percentage of positive cells was calculated. Immunoreactivity scores of CD44 and CD133 in tissue sections were negative (0) in no positive cell in the tumor, weak (+1) when less than 30% of the tumor cells were positive, moderate (+2) whenabout 30%-60% of the tumor cells are positive and strong (+3) when more than 60% of the tumor cells were positive [19].
2.4. Statistical Analysis of Data
- After microscopic examination of H & E slides, prepared immunohistochemistry slides and the qualitative attribute of the expression of CD44 and CD133 markers in each patient, the data were analyzed using SPSS 16.0 software. These analyzes were performed using Fisher's, Chi-square, Spearman correlation coefficient and linear regression (P<0.05).
3. Results
3.1. Expression of CD44 and CD133 in Human Glioma Tissue
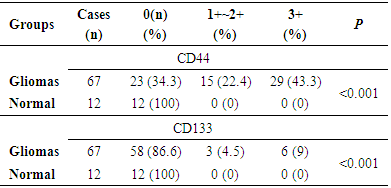
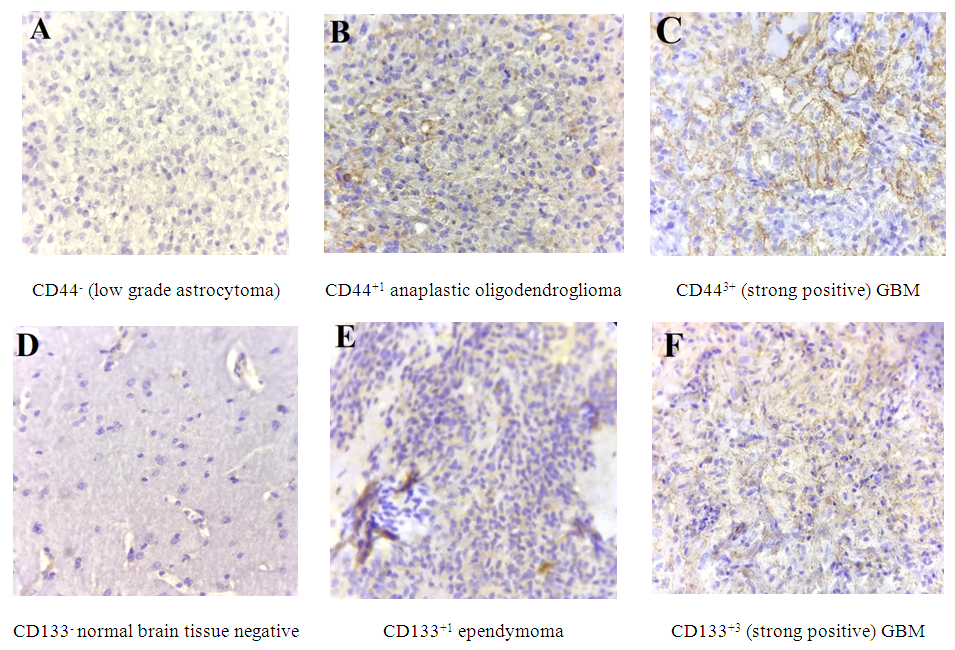
- Anti-CD44 and Anti-CD133 expression in 67 patients with primary glioma were analyzed using immunohistochemical analysis. The positive expression rates of CD44 (44/67, 65.67%) and CD133 (9/67, 13.43%) in patients with gliomas were higher than those in normal brain tissues (0/12, 0%) significantly (P<0.01) (Table 1). These two markers were expressed in the cell membrane and cytoplasm that was confirmed by previous studies [8] (Figure 1 A-B). Figures of immunohistochemical staining of CD44 and CD133 markers are shown in Figure 2A-F.
|
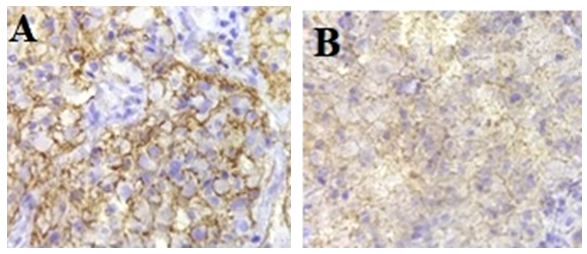 | Figure 1. A. Anti-CD44 expression in stem cells membrane and cytoplasm in GBM, B. Anti-CD133 expression in stem cells membrane in GBM (×100) |
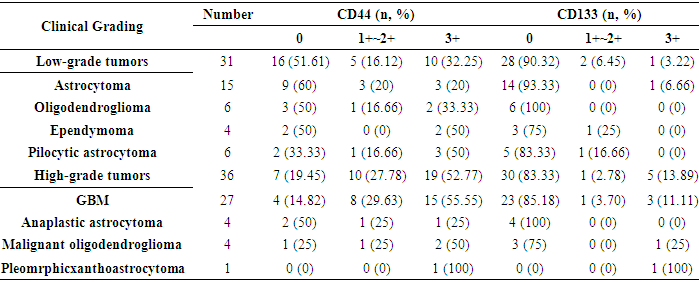
3.2. Correlation between Expression of CD44 and CD133 with Clinical Grading of Human Gliomas
- The expression of CD44 and CD133 markers in human glioma with different clinical grading is shown in Table 2.
|
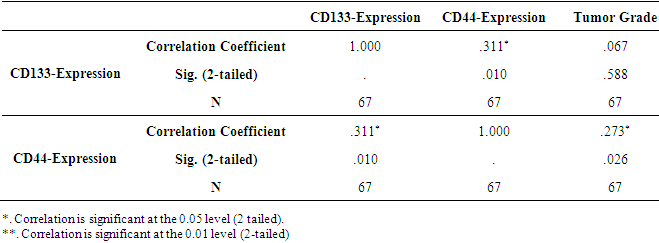
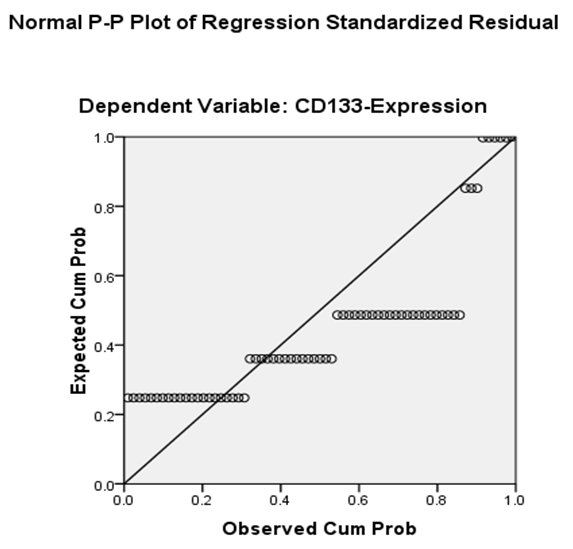 | Figure 3. Correlation between CD44 and CD133 markers expression in studied glial tumors using P-P plot test |
|
|
4. Discussion and Conclusions
- Malignant gliomas are highly recurrent tumors even after surgery, chemotherapy, radiotherapy and immunotherapy. Gliomas treatment strategy has not been tangibly changed by the reason their limited biology finding [20]. In central nervous system tumors, cancer cells differ in their ability to forms tumors. While the majority of the cancer cells have a limited ability to divide, a population of cancer stem cells that has the exclusive ability to extensively proliferate and form new tumors can be identified based on marker expression [21]. However, there is little information about the expression of these markers in brain tumors, especially with association to the malignant grades of these tumors [15]. In order to identify novel prognostic markers in gliomas, several CSC markers have been investigated [6]. Singh et al. (2003) showed that the identification of a brain tumor stem cell (BTSC) provides a powerful tool to investigate the tumorigenic process in the central nervous system and to develop therapies targeted to the BTSC [22]. This study was undertaken to explore the expression of stem cell marker CD44 and CD133 in human gliomas. In our study, CD44 expression was significantly correlated with the histological grade of the tumor, but there was no significant correlation between the expression of the CD133 marker and the histological grading of the tumor (Table 3). The expression of the CD133 stem cell marker may be related to the proliferation of glioma tumor cells and mesenchymal invasion (23). The expression of CD133 marker in grade IV glial tumors was higher than that of grade II tumors [17]. However, in other studies, there was no association between CD133 expression and tumor grade and its outcome, which may be due to differences in the methods of conducting studies [17, 24]. In Table 1 and Figure 2, the expression of the CD133 marker is 9% strong and 4.5% moderate and weak. This marker does not express in normal brain tissue. As Table 2 shows, the expression level of CD133 is only 11.11% of the glioblastomas. In Table 3, there is no significant correlation between histologic grade of tumor and CD133 expression. Wu et al 2016 studies showed no correlation between CD133 protein expression and patient’s survival. Also no correlation between CD133 protein expression and CD133 promoter methylation status was found [25]. CD44 is a glycoprotein marker that is expressed in a variety of malignancies [26]. Xu et al (2010) found that in GBM xenograft models, knockdown of CD44 inhibited cell growth and improved response to chemotherapy [27]. The CD44 marker is strongly expressed in mesenchymal stem cells [28]. As shown in Table 1 and Figure 2, the expression of the CD44 marker was 43.3% strong and 22.4% weak and moderate in gliomas. This marker is not expressed in the normal tissue of the brain. In table 2, the expression of the CD44 marker is shown in a variety of gliomas, the highest of which is 55.55%, in the glioblastoma multiforme. Table 3 shows a significant correlation between the expression of CD44 marker and tumor grade. As shown in Table 4-4 and Figure 3, there is a consistent and non-linear correlation between the expression of CD44 and CD133 markers in glial tumors of the study.Zhang et al (2008) studies using immunohistochemistry method showed that expression level of anti-Nestin and CD133 markers increased significantly with increased glioma tumor grade (P<0.05). There was a positive correlation between expression of these two markers in different glioma tissues (r=0.89). Therefore, the expression of CD133 and Nestin can be suggested as an important feature of human glial tumors, and simultaneous finding of Nestin and CD133 in predicting the invasive nature of this tumor is useful [15]. In Ma et al. (2007) study, the expression of CD133, Nestin, SOX-2, Musashi-1, CXCR4, Flt4 and Endoglin markers on the protein and mRNA levels were used to determine the association between malignancy degree of different types of astrocytoma and the expression of these genes in proliferating glial cells. CD133+ cells were seen with higher levels (15.6%) in astrocytomas with WHO grade IV and 2.3% in normal brain tissue that associated with malignant grades in these tumors. Results in Real Time RT-PCR test were reported as 12.5% in grade IV astrocytoma and 8.5% in normal brain tissue [3]. Also Dalhort et al (2014) using fluorescence staining method reported that CD133 expression was not associated with WHO grade and overall survival rate of patients. Positive expression of the Nestin marker was associated with the glioma WHO grade. Patients expressing high levels of CD133 and Nestin showed low survival [29]. The use of different CD133 antibodyclones possibly recognizing different CD133 splice variants with frail epitopes of differentglycosylation status may explain the different findings. It is therefore most likely the distribution of certain CD133 antigens and not the distribution of the CD133 protein itself that is of prognostic value [6].An attempt has been made to find alternative methods for the isolation of cancer stem cells. Stem cell therapies and isolation and nutritional methods for cancer stem cells continue to be a major challenge [9]. The expressions of CD133, Olig2 and CD44 are correlated with the proliferative or invasive states of a GBM cell. Proliferation in turn correlates with sensitivity to therapy. By monitoring the changes in cellular phenotype using CD133/Olig2 and CD44 markers in the clinic, an improved therapeutic regimen could be designed to minimize acquisition of non-genetic tumor resistance [11].In this study, the relationship between CD44 and glioma tumor grade was significant. This marker looks helpful in predicting tumor grade when tissue specimen is small and all the criteria of grade III or IV tumor cannot be found. Using alternate methods for the isolation of cancerous and non cancerous stem cells and determining of CD44 and CD133 expression levels in other stem cell markers can be suggested in gliomas comparing with normal brain tissues.
ACKNOWLEDGEMENTS
- Authors would like to thank the supervisor and technicians of Department of Pathology in Tehran Firoozgar Hospital, Iran for their helps.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML