| [1] | Koutsogiannouli E, Papavassiliou AG, Papanikolaou NA. Complexity in cancer biology: is systems biology the answer? Cancer Med. Wiley-Blackwell; 2013; 2: 164–77. doi:10.1002/cam4.62. |
| [2] | Du W, Elemento O. Cancer systems biology: embracing complexity to develop better anticancer therapeutic strategies. Oncogene. 2015; 34: 3215–3225. doi: 10.1038/onc.2014.291. |
| [3] | Félix M-A, Barkoulas M. Pervasive robustness in biological systems. Nat Rev Genet. 2015; 16: 483–496. doi: 10.1038/nrg3949. |
| [4] | Balmain A, Gray J, Ponder B. The genetics and genomics of cancer. Nat Genet. 2003; 33: 238–244. doi:10.1038/ng1107. |
| [5] | O’Connor MJ. Targeting the DNA Damage Response in Cancer. Mol Cell. Elsevier; 2015; 60: 547–60. doi:10.1016/j.molcel.2015.10.040. |
| [6] | van Vugt MATM, Reinhardt HC. Editorial: Cancer-Associated Defects in the DNA Damage Response: Drivers for Malignant Transformation and Potential Therapeutic Targets. Front Genet. 2015;6. doi: 10.3389/fgene.2015.00355. |
| [7] | Goldstein M, Kastan MB. The DNA Damage Response: Implications for Tumor Responses to Radiation and Chemotherapy. Annu Rev Med. 2015; 66: 129–143. doi: 10.1146/annurev-med-081313-121208. |
| [8] | Astolfi L, Ghiselli S, Guaran V, Chicca M, Simoni E, Olivetto E, et al. Correlation of adverse effects of cisplatin administration in patients affected by solid tumours: a retrospective evaluation. Oncol Rep. 2013; 29: 1285–92. doi: 10.3892/or.2013.2279. |
| [9] | Elice F, Rodeghiero F. Side effects of anti-angiogenic drugs. Thromb Res. 2012; 129: S50–S53. doi: 10.1016/S0049-3848(12)70016-6. |
| [10] | Baldo B. Adverse events to monoclonal antibodies used for cancer therapy: Focus on hypersensitivity responses. Oncoimmunology. 2013; 2: e26333. doi: 10.4161/onci.26333. |
| [11] | Andrews S, Holden R. Characteristics and management of immunerelated adverse effects associated with ipilimumab, a new immunotherapy for metastatic melanoma. Cancer Manag Res. Dove Press; 2012; 4: 299–307. doi:10.2147/CMAR.S31873. |
| [12] | DeVita VT, Chu E. A History of Cancer Chemotherapy. Cancer Res. 2008; 68. |
| [13] | Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. NIH Public Access; 2009;9: 338–50. doi: 10.1038/nrc2607. |
| [14] | Ewesuedoa R, Ratain M. Topoisomerase I Inhibitors. Oncologist. 1997; 2: 359–364. |
| [15] | Dwarakanath BS, Khaitan D, Mathur R. Inhibitors of topoisomerases as anticancer drugs: problems and prospects. Indian J Exp Biol. 2004; 42: 649–59. Available: http://www.ncbi.nlm.nih.gov/pubmed/15339028. |
| [16] | Alfarouk KO, Stock C-M, Taylor S, Walsh M, Muddathir AK, Verduzco D, et al. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int. BioMed Central; 2015; 15: 71. doi: 10.1186/s12935-015-0221-1. |
| [17] | Mellor HR, Callaghan R. Resistance to chemotherapy in cancer: a complex and integrated cellular response. Pharmacology. Karger Publishers; 2008; 81: 275–300. doi: 10.1159/000115967. |
| [18] | Ferrara N, Hillan KJ, Gerber H-P, Novotny W. Case history: Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. Nature Publishing Group; 2004; 3: 391–400. doi:10.1038/nrd1381. |
| [19] | Meadows KL, Hurwitz HI. Anti-VEGF therapies in the clinic. Cold Spring Harb Perspect Med. Cold Spring Harbor Laboratory Press; 2012; 2. doi: 10.1101/cshperspect.a006577. |
| [20] | Morris JC, Tan AR, Olencki TE, Shapiro GI, Dezube BJ, Reiss M, et al. Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma. PLoS One. Public Library of Science; 2014; 9: e90353. doi: 10.1371/journal.pone.0090353. |
| [21] | Sheen YY, Kim M-J, Park S-A, Park S-Y, Nam J-S. Targeting the Transforming Growth Factor-β Signaling in Cancer Therapy. Biomol Ther (Seoul). Korean Society of Applied Pharmacology; 2013; 21: 323–31. doi:10.4062/biomolther.2013.072. |
| [22] | Xu W, Yang Z, Lu N, Guggenheim D, Shah M, Rahman R, et al. Molecular targeted therapy for the treatment of gastric cancer. J Exp Clin Cancer Res. BioMed Central; 2016; 35: 1. doi: 10.1186/s13046-015-0276-9. |
| [23] | Lindberg JM, Newhook TE, Adair SJ, Walters DM, Kim AJ, Stelow EB, et al. Co-treatment with panitumumab and trastuzumab augments response to the MEK inhibitor trametinib in a patient-derived xenograft model of pancreatic cancer. Neoplasia. Neoplasia Press; 2014; 16: 562–71. doi: 10.1016/j.neo.2014.06.004. |
| [24] | Gutheil JC, Campbell TN, Pierce PR, Watkins JD, Huse WD, Bodkin DJ, et al. Targeted Antiangiogenic Therapy for Cancer Using Vitaxin: A Humanized Monoclonal Antibody to the Integrin alphavbeta3. Clin Cancer Res. 2000; 6: 3056–3061. |
| [25] | Liu Z, Wang F, Chen X. Integrin alpha(v)beta(3)-Targeted Cancer Therapy. Drug Dev Res. NIH Public Access; 2008; 69: 329–339. doi: 10.1002/ddr.20265. |
| [26] | Salas RN, Finke JH, Rini BI. The intersection of sunitinib with the immunosuppressive microenvironment of renal cell carcinoma: implications for future therapeutics. Target Oncol. Springer-Verlag; 2007; 2: 225–234. doi:10.1007/s11523-007-0064-3. |
| [27] | Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013; 138: 105–15. doi:10.1111/imm.12036. |
| [28] | Fujimura T, Kambayashi Y, Aiba S. Crosstalk between regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs) during melanoma growth. Oncoimmunology. Taylor & Francis; 2012; 1: 1433–1434. doi:10.4161/onci.21176. |
| [29] | Coulson A, Levy A, Gossell-Williams M. Monoclonal Antibodies in Cancer Therapy: Mechanisms, Successes and Limitations. West Indian Med J. The University of the West Indies; 2014;63: 650–4. doi:10.7727/wimj.2013.241. |
| [30] | Farid SS. Process economics of industrial monoclonal antibody manufacture. J Chromatogr B Analyt Technol Biomed Life Sci. 2007; 848: 8–18. doi:10.1016/j.jchromb.2006.07.037. |
| [31] | Rudnick SI, Adams GP. Affinity and avidity in antibody-based tumor targeting. Cancer Biother Radiopharm. Mary Ann Liebert, Inc.; 2009; 24: 155–61. doi:10.1089/cbr.2009.0627. |
| [32] | Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev. NIH Public Access; 2008; 60: 1421–34. doi:10.1016/j.addr.2008.04.012. |
| [33] | Altan M, Burtness B, Arteaga C, Engelman J, Burtness B, Goldwasser M, et al. EGFR-directed antibodies increase the risk of severe infection in cancer patients. BMC Med. BioMed Central; 2015; 13: 37. doi:10.1186/s12916-015-0276-9. |
| [34] | Kollmannsberger C, Soulieres D, Wong R, Scalera A, Gaspo R, Bjarnason G. Sunitinib therapy for metastatic renal cell carcinoma: recommendations for management of side effects. Can Urol Assoc J. Canadian Medical Association; 2007; 1: S41-54. Available: http://www.ncbi.nlm.nih.gov/pubmed/18542784. |
| [35] | Morton RF, Hammond EH. ASCO Provisional Clinical Opinion: KRAS, Cetuximab, and Panitumumab-Clinical Implications in Colorectal Cancer. J Oncol Pract. American Society of Clinical Oncology; 2009; 5: 71–2. doi:10.1200/JOP.0924603. |
| [36] | Tan C, Du X. KRAS mutation testing in metastatic colorectal cancer. World J Gastroenterol. Baishideng Publishing Group Inc; 2012; 18: 5171–80. doi:10.3748/wjg.v18.i37.5171. |
| [37] | El Guerrab A, Bamdad M, Kwiatkowski F, Bignon Y-J, Penault-Llorca F, Aubel C. Anti-EGFR monoclonal antibodies and EGFR tyrosine kinase inhibitors as combination therapy for triple-negative breast cancer. Oncotarget. 2016; doi:10.18632/oncotarget.12037. |
| [38] | Zhang F, Yu Y, Xing L, Chen M. Cetuximab combined with chemotherapy is beneficial for patients with advanced non-small cell lung cancer after EGFR-tyrosine kinase inhibitors failure. Int J Clin Exp Med. e-Century Publishing Corporation; 2015; 8: 16140–8. Available: http://www.ncbi.nlm.nih.gov/pubmed/26629127. |
| [39] | Carrato A, Gómez A, Escudero P, Chaves M, Rivera F, Marcuello E, et al. Panitumumab and irinotecan every 3 weeks is an active and convenient regimen for second-line treatment of patients with wild-type K-RAS metastatic colorectal cancer. Clin Transl Oncol. Springer Milan; 2013; 15: 705–711. doi:10.1007/s12094-012-0993-x. |
| [40] | Van Emburgh BO, Sartore-Bianchi A, Di Nicolantonio F, Siena S, Bardelli A, Adam R, et al. Acquired resistance to EGFR-targeted therapies in colorectal cancer. Mol Oncol. Elsevier; 2014; 8: 1084–94. doi:10.1016/j.molonc.2014.05.003. |
| [41] | Hsu H-C, Thiam TK, Lu Y-J, Yeh CY, Tsai W-S, You JF, et al. Mutations of KRAS/NRAS/BRAF predict cetuximab resistance in metastatic colorectal cancer patients. Oncotarget. 2016; 7: 22257–70. doi:10.18632/oncotarget.8076. |
| [42] | Braig F, März M, Schieferdecker A, Schulte A, Voigt M, Stein A, et al. Epidermal growth factor receptor mutation mediates cross-resistance to panitumumab and cetuximab in gastrointestinal cancer. Oncotarget. 2015; 6: 12035–47. doi: 10.18632/oncotarget.3574. |
| [43] | Petitprez A, Poindessous V, Ouaret D, Regairaz M, Bastian G, Guérin E, et al. Acquired irinotecan resistance is accompanied by stable modifications of cell cycle dynamics independent of MSI status. Int J Oncol. 2013; 42: 1644–53. doi:10.3892/ijo.2013.1868. |
| [44] | Dowling JK, Mansell A. Toll-like receptors: the swiss army knife of immunity and vaccine development. Clin Transl Immunol. Nature Publishing Group; 2016; 5: e85. doi: 10.1038/cti.2016.22. |
| [45] | Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. Frontiers Media SA; 2014; 5: 614. doi: 10.3389/fimmu.2014.00614. |
| [46] | Jack CS, Arbour N, Blain M, Meier U-C, Prat A, Antel JP. Th1 polarization of CD4+ T cells by Toll-like receptor 3-activated human microglia. J Neuropathol Exp Neurol. 2007; 66: 848–59. doi: 10.1097/nen.0b013e3181492a7. |
| [47] | Tugues S, Burkhard SH, Ohs I, Vrohlings M, Nussbaum K, vom Berg J, et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ. Nature Publishing Group; 2015; 22: 237–246. doi: 10.1038/cdd.2014.134. |
| [48] | Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. American Association of Immunologists; 2014; 192: 5451–8. doi: 10.4049/jimmunol.1490019. |
| [49] | Ferrantini M, Capone I, Belardelli F. Interferon-α and cancer: Mechanisms of action and new perspectives of clinical use. Biochimie. 2007; 89: 884–893. doi: 10.1016/j.biochi.2007.04.006. |
| [50] | Lee S, Margolin K. Cytokines in Cancer Immunotherapy. Cancers (Basel). 2011;3: 3856–3893. doi:10.3390/cancers3043856. |
| [51] | June CH. Adoptive T cell therapy for cancer in the clinic. J Clin Invest. American Society for Clinical Investigation; 2007; 117: 1466–76. doi:10.1172/JCI32446. |
| [52] | Houot R, Schultz LM, Elien Marabelle A, Kohrt H. T-cell–based Immunotherapy: Adoptive Cell Transfer and Checkpoint Inhibition. Cancer Immunol Res. 3: 1115–22. doi: 10.1158/2326-6066.CIR-15-0190. |
| [53] | Lim O, Jung MY, Hwang YK, Shin E-C. Present and Future of Allogeneic Natural Killer Cell Therapy. Front Immunol. Frontiers Media SA; 2015; 6: 286. doi: 10.3389/fimmu.2015.00286. |
| [54] | Dahlberg CIM, Sarhan D, Chrobok M, Duru AD, Alici E. Natural Killer Cell-Based Therapies Targeting Cancer: Possible Strategies to Gain and Sustain Anti-Tumor Activity. Front Immunol. Frontiers Media SA; 2015; 6: 605. doi: 10.3389/fimmu.2015.00605. |
| [55] | Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor (CAR) design. Cancer Discov. NIH Public Access; 2013;3: 388–98. doi:10.1158/2159-8290.CD-12-0548. |
| [56] | Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med. NIH Public Access; 2012; 14: 405–15. doi:10.1002/jgm.2604. |
| [57] | Newick K, Moon E, Albelda SM, Topalian S, Drake C, Pardoll D, et al. Chimeric antigen receptor T-cell therapy for solid tumors. Mol Ther — Oncolytics. Nature Publishing Group; 2016; 3: 16006. doi:10.1038/mto.2016.6. |
| [58] | Lutsiak MEC, Semnani RT, De Pascalis R, Kashmiri SVS, Schlom J, Sabzevari H. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005; 105: 2862–2868. |
| [59] | Alizadeh D, Larmonier N. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res. NIH Public Access; 2014; 74: 2663–8. doi: 10.1158/0008-5472.CAN-14-0301. |
| [60] | Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008; 8: 59–73. doi: 10.1038/nri2216. |
| [61] | Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015; 15: 1145–1154. doi: 10.1517/14712598.2015.1046430. |
| [62] | Zhang H, Ye Z-L, Yuan Z-G, Luo Z-Q, Jin H-J, Qian Q-J. New Strategies for the Treatment of Solid Tumors with CAR-T Cells. Int J Biol Sci. Ivyspring International Publisher; 2016; 12: 718–29. doi:10.7150/ijbs.14405. |
| [63] | Prosser ME, Brown CE, Shami AF, Forman SJ, Jensen MC. Tumor PD-L1 co-stimulates primary human CD8+ cytotoxic T cells modified to express a PD1:CD28 chimeric receptor. Mol Immunol. 2012; 51: 263–272. doi: 10.1016/j.molimm.2012.03.023. |
| [64] | Kitano H. Biological robustness. Nat Rev Genet. Nature Publishing Group; 2004; 5: 826–837. doi: 10.1038/nrg1471. |
| [65] | Kreeger PK, Lauffenburger DA. Cancer systems biology: a network modeling perspective. Carcinogenesis. Oxford University Press; 2010; 31: 2–8. doi:10.1093/carcin/bgp261. |
| [66] | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971; 285: 1182–6. doi: 10.1056/NEJM197111182852108. |
| [67] | Xu J, Vilanova G, Gomez H, Folkman J, Hicklin D, Ellis L, et al. A Mathematical Model Coupling Tumor Growth and Angiogenesis. Oberai AA, editor. PLoS One. Public Library of Science; 2016; 11: e0149422. doi: 10.1371/journal.pone.0149422. |
| [68] | Gevertz JL, Torquato S. Modeling the effects of vasculature evolution on early brain tumor growth. J Theor Biol. 2006; 243: 517–31. doi:10.1016/j.jtbi.2006.07.002. |
| [69] | Peirce SM. Computational and mathematical modeling of angiogenesis. Microcirculation. NIH Public Access; 2008;15: 739–51. doi:10.1080/10739680802220331. |
| [70] | Cameron MA, Davis AL, Kostelich E, Eikenberry S. A Mathematical Model of Angiogenesis in Glioblastoma Multiforme [Internet]. Arizona State University. 2009. Available: http://stanford.edu/~mary32/GBM_Paper.pdf. |
| [71] | Soto-Ortiz L, Finley SD. A cancer treatment based on synergy between anti-angiogenic and immune cell therapies. J Theor Biol. 2016; 394: 197–211. doi:10.1016/j.jtbi.2016.01.026. |
| [72] | Robertson-Tessi M, El-Kareh A, Goriely A. A mathematical model of tumor-immune interactions. J Theor Biol. 2012; 294: 56–73. doi:10.1016/j.jtbi.2011.10.027. |
| [73] | Wilkie KP. A review of mathematical models of cancer-immune interactions in the context of tumor dormancy. Adv Exp Med Biol. 2013; 734: 201–34. doi: 10.1007/978-1-4614-1445-2_10. |
| [74] | Eftimie R, Bramson JL, Earn DJD. Interactions Between the Immune System and Cancer: A Brief Review of Non-spatial Mathematical Models. Bull Math Biol. Springer-Verlag; 2011; 73: 2–32. doi:10.1007/s11538-010-9526-3. |
| [75] | Den Breems NY, Eftimie R. The re-polarisation of M2 and M1 macrophages and its role on cancer outcomes. J Theor Biol. 2016; 390: 23–39. |
| [76] | Kogan Y, Agur Z, Elishmereni M. A Mathematical Model for the Immunotherapeutic Control of the Th1/Th2 Imbalance in Melanoma. Dyn Syst Ser B. 2013; 18: 1017–1030. doi:10.3934/dcdsb.2013.18.1017. |
| [77] | Araujo RP, Petricoin EF, Liotta LA. A mathematical model of combination therapy using the EGFR signaling network. Biosystems. 2005; 80: 57–69. doi:10.1016/j.biosystems.2004.10.002. |
| [78] | Callahan MK. Two Drugs Are Better than One—Modeling Drug Combinations in Cancer Therapy. Sci Transl Med. 2013; 5. |
| [79] | Bozic I, Reiter JG, Allen B, Antal T, Chatterjee K, Shah P, et al. Evolutionary dynamics of cancer in response to targeted combination therapy. Elife. eLife Sciences Publications Limited; 2013; 2: e00747. doi: 10.7554/eLife.00747. |
| [80] | Sun X, Bao J, Shao Y, Camidge DR, Pao W, Sequist L V., et al. Mathematical Modeling of Therapy-induced Cancer Drug Resistance: Connecting Cancer Mechanisms to Population Survival Rates. Sci Rep. Nature Publishing Group; 2016; 6: 22498. doi: 10.1038/srep22498. |
| [81] | Tomasetti C, Levy D. An elementary approach to modeling drug resistance in cancer. Math Biosci Eng. NIH Public Access; 2010; 7: 905–18. Available: http://www.ncbi.nlm.nih.gov/pubmed/21077714. |
| [82] | Panetta JC. A Mathematical Model of Drug Resistance: Heterogeneous Tumors. Math Biosci. 1998; 147: 41–61. |
| [83] | De Pillis LG, Radunskaya AE, Wiseman CL. A Validated Mathematical Model of Cell-Mediated Immune Response to Tumor Growth. Cancer Res. 2005; 65: 7950–8. doi: 10.1158/0008-5472.CAN-05-0564. |
| [84] | Kirschner D, Panetta JC. Modeling immunotherapy of the tumor – immune interaction. J Math Biol. 1998; 37: 235–252. |
| [85] | Kim PS, Lee PP, Nestle F, Tonel G, Farkas A, Jaini R, et al. Modeling Protective Anti-Tumor Immunity via Preventative Cancer Vaccines Using a Hybrid Agent-based and Delay Differential Equation Approach. Beerenwinkel N, editor. PLoS Comput Biol. Public Library of Science; 2012; 8: e1002742. doi:10.1371/journal.pcbi.1002742. |
| [86] | dePillis LG, Savage H, Radunskaya AE. Mathematical Model of Colorectal Cancer with Monoclonal Antibody Treatments. Br J Med Med Res. 2014; 4. doi:http://dx.doi.org/10.9734/bjmmr/2014/8393. |
| [87] | Sameen S, Barbuti R, Milazzo P, Cerone A, Del Re M, Danesi R. Mathematical modeling of drug resistance due to KRAS mutation in colorectal cancer. J Theor Biol. 2016; 389: 263–273. doi:10.1016/j.jtbi.2015.10.019. |
| [88] | Arciero JC, Jackson TL, Kirschner De. a Mathematical Model of Tumor-Immune Evasion and Sirna Treatment. Discret Contin Dyn Syst - Ser B. 2004;4: 39–58. Available: http://aimsciences.org. |
| [89] | DePillis L, Caldwell T, Sarapata E, Williams H. Mathematical modeling of regulatory T cell effects on renal cell carcinoma treatment. Discret Contin Dyn Syst - Ser B. 2013; 18: 915–943. doi:10.3934/dcdsb.2013.18.915. |
| [90] | Liu C-Z, Zhang L, Chang X-H, Cheng Y-X, Cheng H-Y, Ye X, et al. Overexpression and immunosuppressive functions of transforming growth factor 1, vascular endothelial growth factor and interleukin-10 in epithelial ovarian cancer. Chin J Cancer Res. Beijing Institute for Cancer Research; 2012; 24: 130–7. doi:10.1007/s11670-012-0130-y. |
| [91] | De Pillis LG, Radunskaya A. A mathematical model of immune response to tumor invasion. Second MIT Conference on Computational Fluid and Solid Mechanics. 2003. pp. 1661–1668. |
| [92] | Sakamoto N, Ishikawa T, Kokura S, Okayama T, Oka K, Ideno M, et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med. BioMed Central; 2015; 13: 277. doi:10.1186/s12967-015-0632-8. |
| [93] | Kronik N, Kogan Y, Vainstein V, Agur Z. Improving alloreactive CTL immunotherapy for malignant gliomas using a simulation model of their interactive dynamics. Cancer Immunol Immunother. 2008; 57: 425–39. doi: 10.1007/s00262-007-0387-z |
| [94] | Kim KS, Cho G, Jung IH. Optimal treatment strategy for a tumor model under immune suppression. Comput Math Methods Med. 2014;2014: 206287. doi: 10.1155/2014/206287. |
| [95] | Welink J, Boven E, Vermorken JB, Gall HE, van der Vijgh WJ. Pharmacokinetics and pharmacodynamics of lobaplatin (D-19466) in patients with advanced solid tumors, including patients with impaired renal of liver function. Clin Cancer Res. 1999; 5: 2349–58. Available: http://www.ncbi.nlm.nih.gov/pubmed/10499604. |
| [96] | Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. NIH Public Access; 2007; 25: 267–96. doi:10.1146/annurev.immunol.25.022106.141609. |
| [97] | de Pillis L, Fister KR, Gu W, Collins C, Daub M, Gross D, et al. Mathematical Model Creation for Cancer Chemo-Immunotherapy. Comput Math Methods Med. Hindawi Publishing Corporation; 2009; 10: 165–184. doi:10.1080/17486700802216301. |
| [98] | Bian X, Jiang X, Chen J, Bai J, Dai C, Wang Q, et al. Increased angiogenic capabilities of endothelial cells from microvessels of malignant human gliomas. Int Immunopharmacol. 2006; 6: 90–9. doi:10.1016/j.intimp.2005.08.004. |
| [99] | Hahnfeldt P, Panigrahy D, Folkman J, Hlatky L. Tumor development under angiogenic signaling: a dynamical theory of tumor growth, treatment response, and postvascular dormancy. Cancer Res. 1999; 59: 4770–5. Available: http://www.ncbi.nlm.nih.gov/pubmed/10519381. |
| [100] | Mabarrack NHE, Turner NL, Mayrhofer G. Recent thymic origin, differentiation, and turnover of regulatory T cells. J Leukoc Biol. 2008; 84: 1287–97. doi:10.1189/jlb.0308201. |


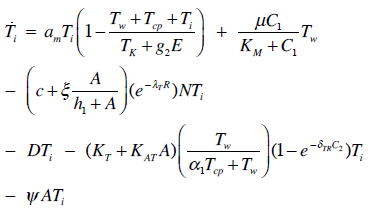
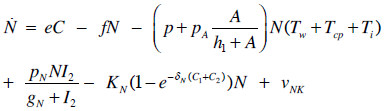
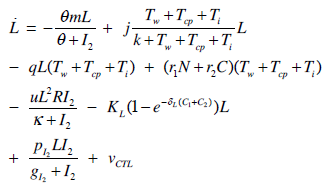
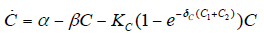
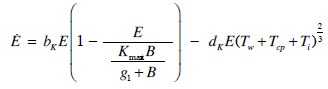
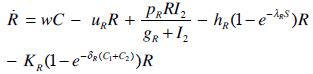
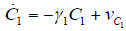
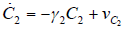
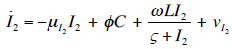
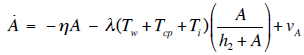
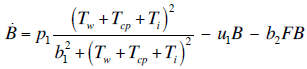
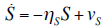
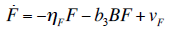
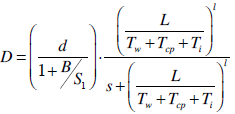
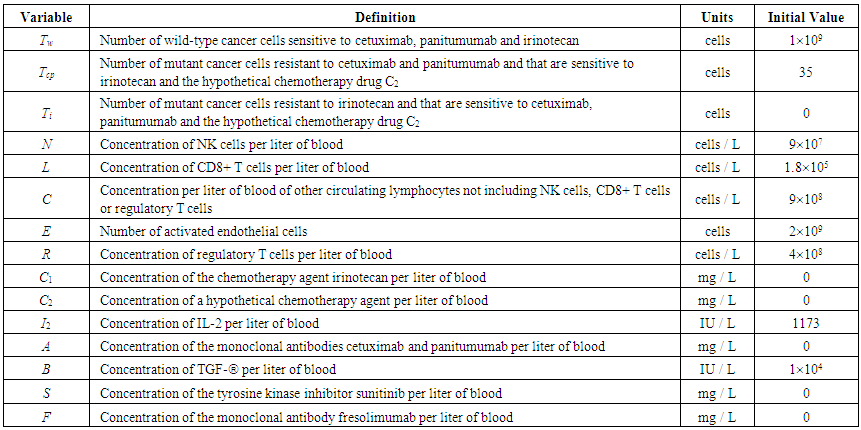
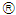 and anti-TGF-
and anti-TGF-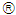 were converted from mg/L to IU/L, and vice versa, by referring to the published specific activity of TGF-
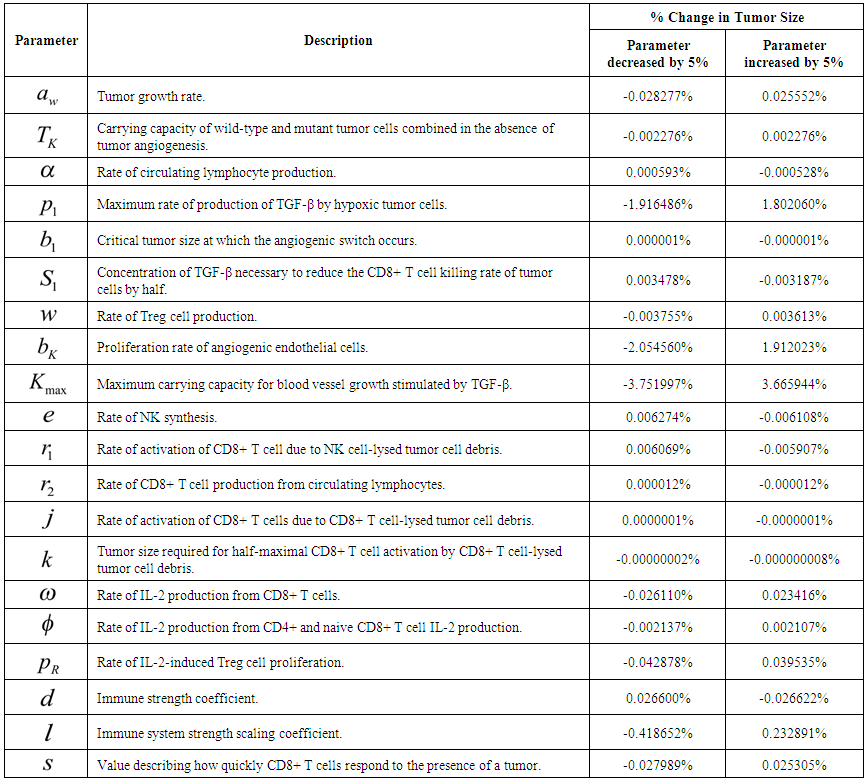
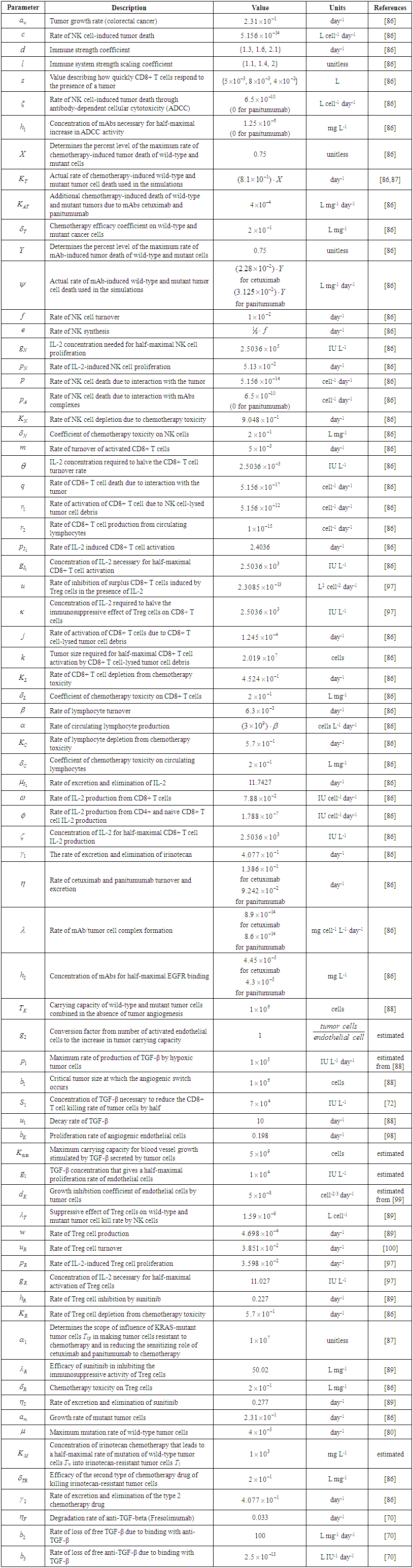
were converted from mg/L to IU/L, and vice versa, by referring to the published specific activity of TGF-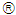 . An example of how to perform this conversion can be found in [94].The parameters d, l and s together determine the level of strength of a patient’s immune system response D, as defined by equation 15. The values of these parameters are taken from the sets
. An example of how to perform this conversion can be found in [94].The parameters d, l and s together determine the level of strength of a patient’s immune system response D, as defined by equation 15. The values of these parameters are taken from the sets 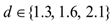 ,
, 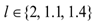 and
and 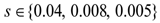 . A strong immune response (Ds) corresponds to the combination of values
. A strong immune response (Ds) corresponds to the combination of values 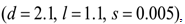 a moderate response (Dm) corresponds to the values
a moderate response (Dm) corresponds to the values 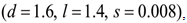 and a weak response (Dw) is characterized by the values
and a weak response (Dw) is characterized by the values 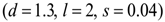 . These levels of immune strength are the same as those that were considered in [86].The anti-cancer treatments listed in Table 2, as well as certain combinations of them, were assessed for their robustness with respect to the three levels of immune strength Ds, Dm, and Dw. To assess the robustness of a treatment with respect to tumor size, initial tumor sizes of up to 109 cells were considered. Robustness with respect to resistance to therapy was assessed by comparing two cases: 1) a case where wild-type tumor cells cannot become resistant to any treatment and there are no mutant cells present at the start of treatment, and 2) a case where there are 35 KRAS-mutant tumor cells initially present, and there are no irinotecan-resistant cells present, but irinotecan resistance emerges in an irinotecan dose-dependent manner. The emergence of irinotecan resistance is represented by the term
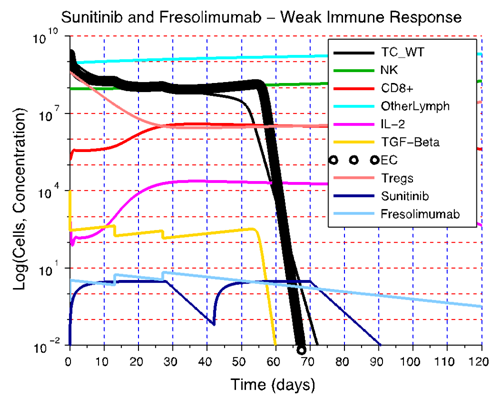
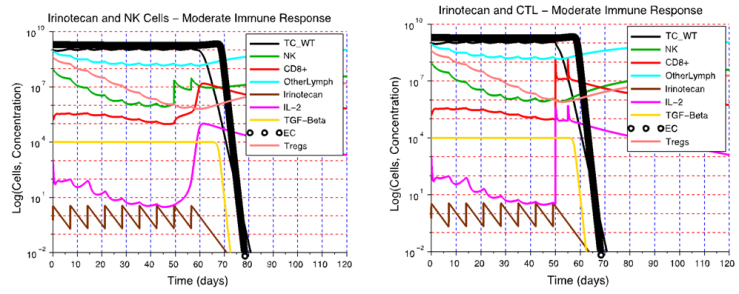
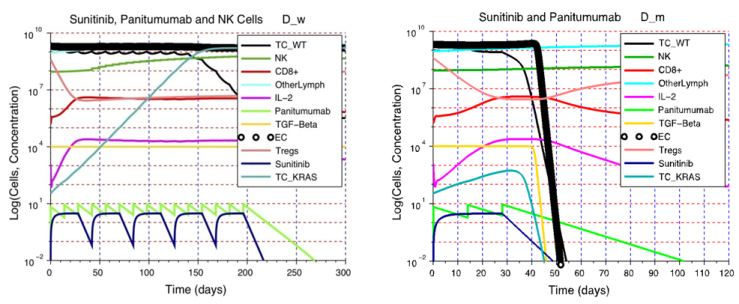
. These levels of immune strength are the same as those that were considered in [86].The anti-cancer treatments listed in Table 2, as well as certain combinations of them, were assessed for their robustness with respect to the three levels of immune strength Ds, Dm, and Dw. To assess the robustness of a treatment with respect to tumor size, initial tumor sizes of up to 109 cells were considered. Robustness with respect to resistance to therapy was assessed by comparing two cases: 1) a case where wild-type tumor cells cannot become resistant to any treatment and there are no mutant cells present at the start of treatment, and 2) a case where there are 35 KRAS-mutant tumor cells initially present, and there are no irinotecan-resistant cells present, but irinotecan resistance emerges in an irinotecan dose-dependent manner. The emergence of irinotecan resistance is represented by the term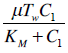 that appears in equations 1 and 3.Cancer therapy tends to have side effects that must be kept into consideration when designing a treatment protocol. Chemotherapy has the undesirable effect of killing a significant number of immune cells. Cetuximab and panitumumab lead to NK cell death due to their interaction with mAb-tumor complexes. To ensure the safety in the clinic of all the simulated treatments that led to a significant reduction in tumor size, the criteria for minimum circulating lymphocyte concentration of
that appears in equations 1 and 3.Cancer therapy tends to have side effects that must be kept into consideration when designing a treatment protocol. Chemotherapy has the undesirable effect of killing a significant number of immune cells. Cetuximab and panitumumab lead to NK cell death due to their interaction with mAb-tumor complexes. To ensure the safety in the clinic of all the simulated treatments that led to a significant reduction in tumor size, the criteria for minimum circulating lymphocyte concentration of 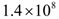 lymphocytes per liter set by the World Health Organization (WHO) [95] was enforced. Depending on the effectiveness of a particular treatment, the predicted outcome was the elimination of the tumor, reduced or arrested growth, or continued growth. In all the simulations, a complete response (CR) to a cancer treatment was defined as a tumor size at the end of the treatment period that is less than or equal to the diffusion-limited value of
lymphocytes per liter set by the World Health Organization (WHO) [95] was enforced. Depending on the effectiveness of a particular treatment, the predicted outcome was the elimination of the tumor, reduced or arrested growth, or continued growth. In all the simulations, a complete response (CR) to a cancer treatment was defined as a tumor size at the end of the treatment period that is less than or equal to the diffusion-limited value of 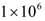 tumor cells. Hence, a complete response is characterized by a reduction in tumor size below the threshold that would trigger the angiogenic switch. A partial response (PR) to treatment is described by a tumor that remains larger than
tumor cells. Hence, a complete response is characterized by a reduction in tumor size below the threshold that would trigger the angiogenic switch. A partial response (PR) to treatment is described by a tumor that remains larger than 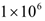 cells, but that by the end of the treatment period is smaller than at the start of treatment. The no response (NR) classification applies when tumor size remains the same, or becomes larger than it was at the start of treatment, by the time the treatment period ends. A cancer treatment that leads to a complete response (CR) at the three levels of immune response strength D, for tumor sizes of up to 109 cells at the start of treatment, does not lead to the emergence of treatment resistance and is effective in spite of resistant cells being present, and that meets the minimum lymphocyte concentration criteria for safety of at least
cells, but that by the end of the treatment period is smaller than at the start of treatment. The no response (NR) classification applies when tumor size remains the same, or becomes larger than it was at the start of treatment, by the time the treatment period ends. A cancer treatment that leads to a complete response (CR) at the three levels of immune response strength D, for tumor sizes of up to 109 cells at the start of treatment, does not lead to the emergence of treatment resistance and is effective in spite of resistant cells being present, and that meets the minimum lymphocyte concentration criteria for safety of at least 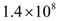 circulating lymphocytes per liter throughout the treatment period, is defined as being robust with respect to the above perturbations. This is the definition of robustness of an anti-cancer treatment that was considered in the computational analysis presented in the next section.
circulating lymphocytes per liter throughout the treatment period, is defined as being robust with respect to the above perturbations. This is the definition of robustness of an anti-cancer treatment that was considered in the computational analysis presented in the next section.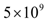 EC. It was also assumed that each endothelial cell that becomes activated increases the tumor carrying capacity by 1 tumor cell. Hence, an angiogenic switch allows a simulated tumor to increase its carrying capacity by up to
EC. It was also assumed that each endothelial cell that becomes activated increases the tumor carrying capacity by 1 tumor cell. Hence, an angiogenic switch allows a simulated tumor to increase its carrying capacity by up to 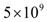 tumor cells.
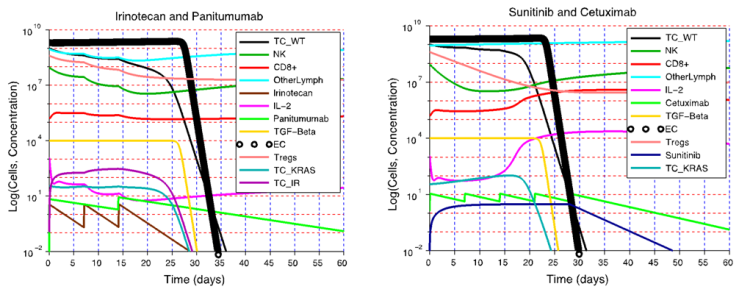
tumor cells. 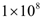 cells will be eliminated without the need for treatment. This outcome represents the ideal case, where a body’s own defenses eliminate an infection without the need for medical intervention. The decrease in the number of activated endothelial cells (EC), shown in all the figures as a thick black line, is due to a decrease in TGF-β secreted by tumor cells. As immune cells continue to kill tumor cells, the concentration of TGF-β decreases, leading to endothelial cell deactivation and a decrease in the carrying capacity of activated ECs and of tumor cells. The thin black line represents the actual number of tumor cells over time.The therapeutic tumor size threshold corresponding to a moderate strength Dm is predicted to be approximately
cells will be eliminated without the need for treatment. This outcome represents the ideal case, where a body’s own defenses eliminate an infection without the need for medical intervention. The decrease in the number of activated endothelial cells (EC), shown in all the figures as a thick black line, is due to a decrease in TGF-β secreted by tumor cells. As immune cells continue to kill tumor cells, the concentration of TGF-β decreases, leading to endothelial cell deactivation and a decrease in the carrying capacity of activated ECs and of tumor cells. The thin black line represents the actual number of tumor cells over time.The therapeutic tumor size threshold corresponding to a moderate strength Dm is predicted to be approximately 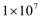 cells. Figure 3B shows that if a tumor has a size that is above this threshold, a compromised immune system capable of only a moderate anti-tumor response will not be able to arrest tumor growth. The tumor will eventually attain a size equal to its dynamic carrying capacity. This escape from immunodestruction by the tumor is due to a tip of the balance between the strength of the anti-tumor immune response and immunosuppression exerted by Treg cells in favor of immunosuppression. Figure 3C illustrates the prediction that if the immune response is weak (Dw), only tumors that have a size equal to or smaller than the diffusion-limited size of 1 million cells will be destroyed by the immune system. Hence, in the absence of treatment, an immune system that exhibits a weak anti-tumor response can only eliminate avascular tumors.
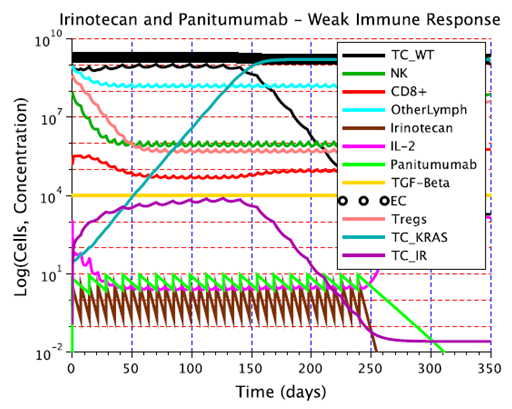
cells. Figure 3B shows that if a tumor has a size that is above this threshold, a compromised immune system capable of only a moderate anti-tumor response will not be able to arrest tumor growth. The tumor will eventually attain a size equal to its dynamic carrying capacity. This escape from immunodestruction by the tumor is due to a tip of the balance between the strength of the anti-tumor immune response and immunosuppression exerted by Treg cells in favor of immunosuppression. Figure 3C illustrates the prediction that if the immune response is weak (Dw), only tumors that have a size equal to or smaller than the diffusion-limited size of 1 million cells will be destroyed by the immune system. Hence, in the absence of treatment, an immune system that exhibits a weak anti-tumor response can only eliminate avascular tumors. 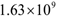 panitumumab-resistant cells, leading to treatment failure.
panitumumab-resistant cells, leading to treatment failure.
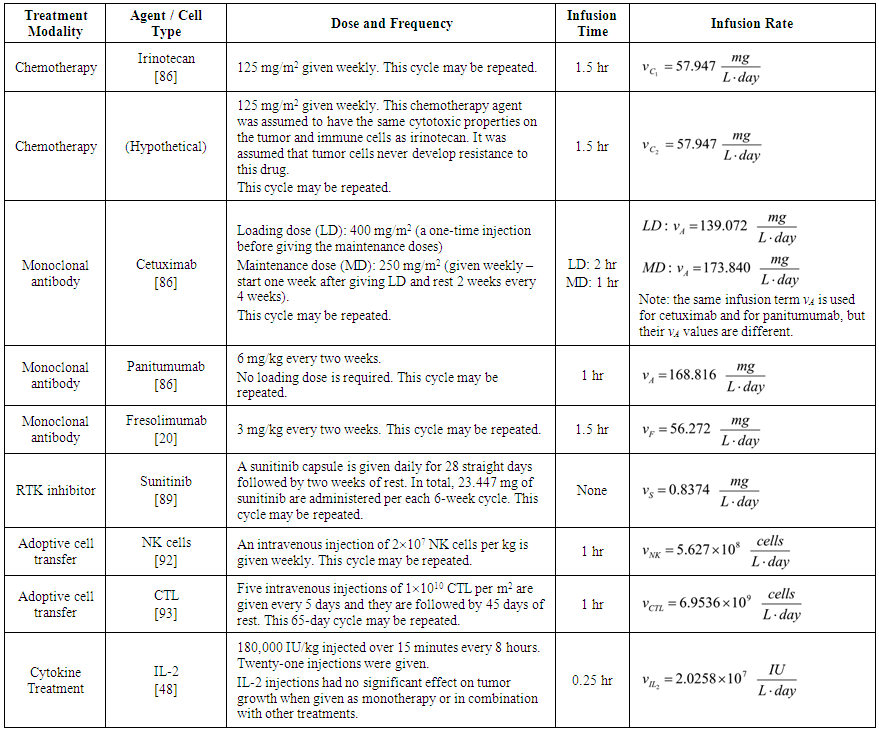
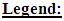 I = IrinotecanC = CetuximabP = PanitumumabS = SunitinibF = FresolimumabNK = Natural killer cellsCTL = Cytotoxic T lymphocytes C2 = Hypothetical chemotherapy drug
I = IrinotecanC = CetuximabP = PanitumumabS = SunitinibF = FresolimumabNK = Natural killer cellsCTL = Cytotoxic T lymphocytes C2 = Hypothetical chemotherapy drug CR = Complete response: a tumor size at the end of the treatment period that is less than or equal to the diffusion-limited value of 1106 tumor cells.PR = Partial response: a tumor that remains larger than 1106 cells, but that by the end of the treatment period is smaller than at the start of treatment.NR = No response: the tumor size remains the same, or becomes larger than it was at the start of treatment, by the time the treatment period ends.
CR = Complete response: a tumor size at the end of the treatment period that is less than or equal to the diffusion-limited value of 1106 tumor cells.PR = Partial response: a tumor that remains larger than 1106 cells, but that by the end of the treatment period is smaller than at the start of treatment.NR = No response: the tumor size remains the same, or becomes larger than it was at the start of treatment, by the time the treatment period ends.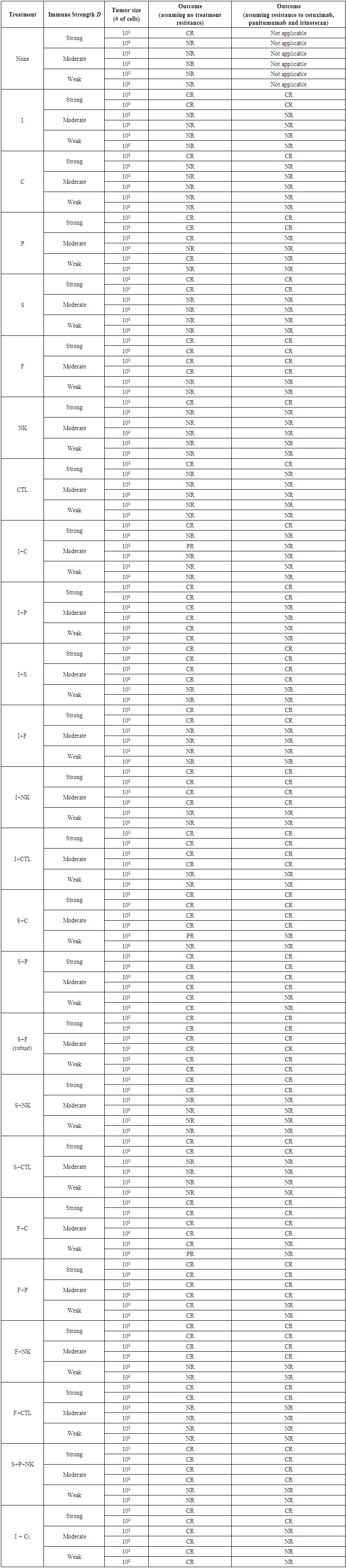
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML
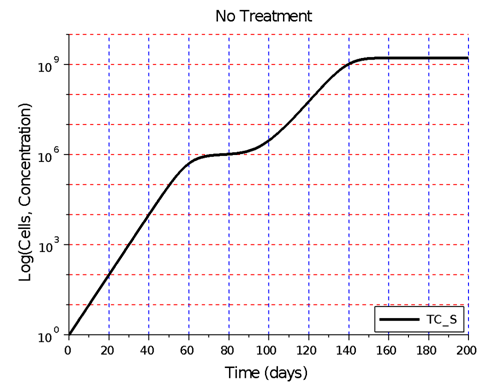
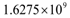 cells on day 200. The figure illustrates a tumor dormancy of approximately one week. The dormancy period may be longer or may be nonexistent, depending on the environmental conditions and the type of tumor
cells on day 200. The figure illustrates a tumor dormancy of approximately one week. The dormancy period may be longer or may be nonexistent, depending on the environmental conditions and the type of tumor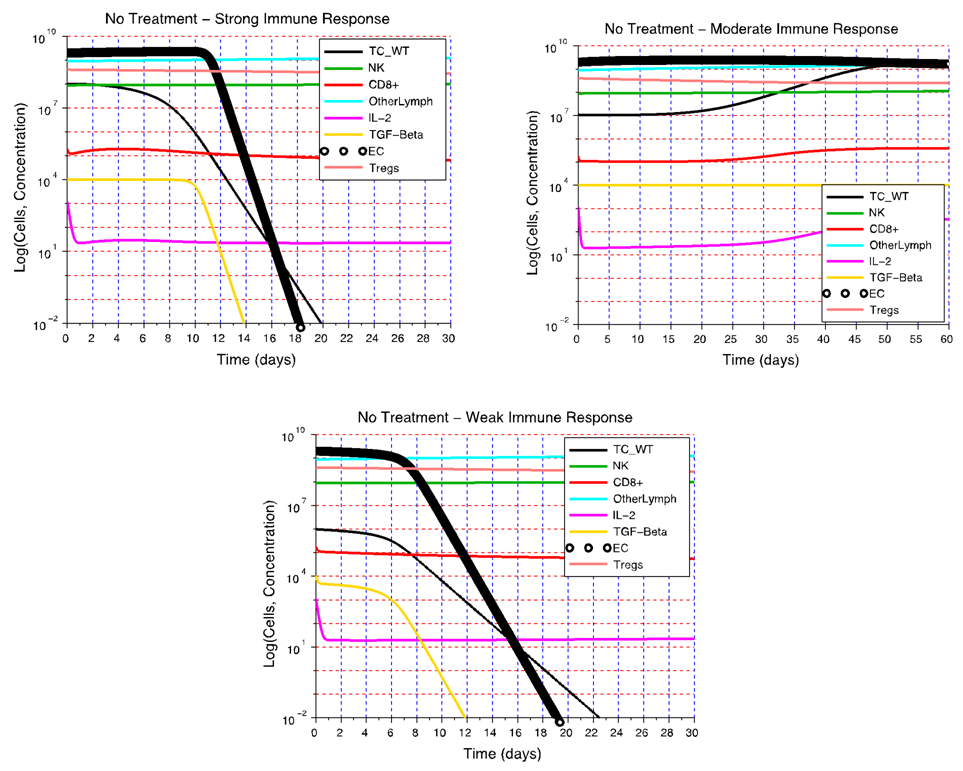
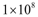 cells. B) Without treatment, a moderate immune response Dm is not able to eliminate a tumor that is larger than
cells. B) Without treatment, a moderate immune response Dm is not able to eliminate a tumor that is larger than 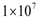 cells. C) If the immune response is weak (Dw), only non-vascularized tumors consisting of less than 1 million cells will be eliminated in the absence of treatment
cells. C) If the immune response is weak (Dw), only non-vascularized tumors consisting of less than 1 million cells will be eliminated in the absence of treatment