-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Tumor Therapy
p-ISSN: 2163-2189 e-ISSN: 2163-2197
2016; 5(1): 1-7
doi:10.5923/j.ijtt.20160501.01

Protracted Half-Body Irradiation Instead of Chemotherapy: Life Span and Lymphocytopenia in Relapsed Ovarian Cancer
Aleksey N. Shoutko1, Ludmila E. Yurkova2, Kseniya S. Borodulya2, Ludmila P. Ekimova1
1Laboratory for Improvement of the Treatment Methods, Federal Research Centre for Radiology and Surgical Technologies, Saint-Petersburg, Russian Federation
2Department of Radiology, Federal Research Centre for Radiology and Surgical Technologies, Saint-Petersburg, Russian Federation
Correspondence to: Aleksey N. Shoutko, Laboratory for Improvement of the Treatment Methods, Federal Research Centre for Radiology and Surgical Technologies, Saint-Petersburg, Russian Federation.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Purpose: Greater understanding of the role of half-body irradiation (HBI) in the treatment of advanced cancer could be achieved by comparison of prolonged HBI with conventional chemotherapy at different levels of baseline lymphocytopenia. Methods: Twelve patients with relapsed ovarian epithelial cancer (OC) received four courses of lower-HBI (0.1 Gy ×10 daily) followed by four courses of chemotherapy with carboplatinum and docetaxel. Sixteen patients received only eight courses of a similar chemotherapy regimen. Both therapies were continued for 10 months and were followed by monitoring of peripheral blood lymphocytes. Results: The average life spans (LSs) were maximal (42.6 months with HBI and 29.3 months with chemotherapy) at lymphocytopenia levels after 10 months of treatment (Lph10) of approximately 1.3×109/L and 1.5×109/L, respectively. The LS was less than optimal when influenced by stronger post-treatment lymphocytopenia after HBI in the subgroup with low baseline Lph0 or by weaker lymphocytopenia after chemotherapy in the subgroup with normal baseline Lph0. Conclusions: Longitudinal HBI contributed to longevity not less, than conventional chemotherapy, depending directly on the optimal level of lymphocytopenia achieved at 10 months after treatment initiation. Optimal level needs to be balanced with the hematopoietic resources of the patient, independently of the type of systemic cytotoxic treatment.
Keywords: HBI- half-body irradiation, OC- ovarian carcinoma, LS- life span, Lph-lymphocytes number per liter of a blood
Cite this paper: Aleksey N. Shoutko, Ludmila E. Yurkova, Kseniya S. Borodulya, Ludmila P. Ekimova, Protracted Half-Body Irradiation Instead of Chemotherapy: Life Span and Lymphocytopenia in Relapsed Ovarian Cancer, International Journal of Tumor Therapy, Vol. 5 No. 1, 2016, pp. 1-7. doi: 10.5923/j.ijtt.20160501.01.
Article Outline
1. Introduction
- Augmentation of the immune response by low-dose total body irradiation (TBI) or half-body irradiation (HBI) has been proposed as a possible reason for the benefit achieved by treatment with fractionated exposure up to a total dose of 1.5 Gy during 5 weeks in patients with chronic lymphocytic leukemia (CLL) and non-Hodgkin’s lymphoma (NHL) [1, 2].Previously, the need for or utility of limited myelosuppression was suggested in [3, 4], and one course of HBI (0.1 Gy×10 times for 3 weeks or 3 Gy×3 times daily) was used successfully for the treatment of advanced ovarian carcinoma (OC) to assess the therapeutic efficacy of low doses of external radiation [5]. For many reasons, the idea of immune response enhancement by low-dose irradiation is not universally accepted at present. A unidirectional change in cellular immunity might slow down tumor progression in some patient groups but be detrimental in others [6]. Lymphocytopenia before treatment is a negative prognostic sign for cancer treatment [7], whereas limited leucopenia during chemoradiotherapy is associated with improved overall survival [8-11]. The ability of bone marrow-derived circulating cells to support regeneration in different tissues including malignant ones [3, 4, 12-14] may explain these and other inconsistencies. It provides a mechanism based on the inactivation of such morphogenic cells due to cytotoxic treatment. This alternative mechanism compromises the idea of antitumor immunity and immune enhancement arising in parallel with post-therapeutic myelosuppression. Moreover, it challenges the idea of radiation hormesis, which is also based on immune enhancement [15].In the chronic TBI of dogs (range, 0.0003-0.026 Gy daily), both the rate of solid cancers and life span (LS) were higher in animals with normal basal bone marrow (BM) function compared with those that had congenital weakness of basal myelopoiesis [16]. Any agent that provoked myelosuppression quantitatively reduced the feeding influence of circulating cells from the bone marrow on the tumor.Reparable damage in the most rapidly renewing cells in bone marrow and other normal tissues chemoattracted the rest of the circulating feeding cells, thereby weakening their participation in tumor growth maintenance. The lowering of the number of feeding cells and their competitive relocation from the tumor to the multitude of sublethally affected normal cells may overlap with each other, depending on the power of systemic radiotherapy or chemotherapy and on the current potency/capacity of the BM to selfregenerate [11, 17-20]. The most effective feeding cells (CD133+ angiogenic stem cells, CD34+ progenitors, TdT+ pre-lymphocytes, certain other “regulatory” cells, and CD31+ T- angiogenic lymphocytes) are mononuclear. They all concentrate mainly in the common fraction of blood lymphocytes. From this point of view, the high sensitivity of lymphocytopoiesis to radiation injury provokes the crucial question of why HBI or TBI is not an alternative treatment to chemotherapy. ObjectivesThus, the primary goal of our study was to evaluate the effectiveness of several courses of HBI compared with chemotherapy in relapsed OC in terms of lymphocytopenia and LS. Because the basal level of lymphocytes in blood might reflect the degree of the BM’s regenerative potential, two groups of patients with different average values of this baseline parameter were tested.
2. Subjects and Methods
2.1. Subjects
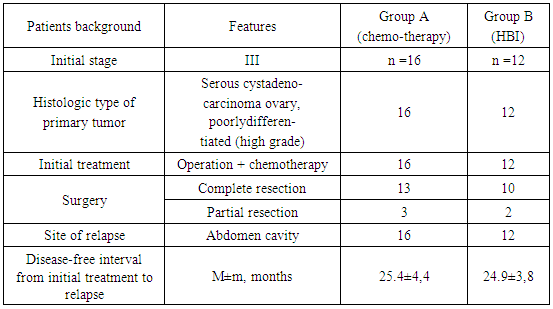
- In total, 28 patients with relapsed epithelial OC were attending the RRCRST for treatment between the winters of 2000 and 2010. Informed consent was obtained from all participants of the trial. The age of the subjects ranged from 40 to 58 years. Patients were divided into two groups. A brief introduction about the patients is given on Table 1.
|
2.2. Therapy
- Group A included 16 patients (average age 51 years) who received eight courses of conventional chemotherapy with carboplatinum and docetaxel during the first 10 months. Each course included 1cycle of docetaxel 75 mg/m2 by 1-hour infusion intravenous followed by carboplatin infusion during next 1 hour. Doses of carboplatin (from 400 to 505 mg was calculated according Calvert formula based on creatinine urine clearance measured 24 hour for each cycle [21]. Premedication with dexamethasone 20 mg twice was begun 12 hours and repeated 6 hours before infusion of docetaxel Consecutive infusions of H1 blocker ranitidine (zantac, 50 mg in 100 ml, over 30 minutes) and H2 blocker diphenhydramine (dimedrol, 50mg in 100 ml, over 30 minutes) were performed just before docetaxel infusion.Twelve patients in Group B (average age 49 years) received four courses of HBI followed by four identical courses of chemotherapy. The HBI technology was developed in the 1990s for patients with gastrointestinal tumors, Ewing’s sarcoma, breast cancer, and carcinomas of the lungs and ovaries [5, 22]. Irradiation was performed for 10 months up to a total dose of 4 Gy using X-rays produced by a 6-MeV linear accelerator. Each course included 10 fractions of 0.1 Gy daily, administered to the lower part of the body with the dome of the abdominal diaphragm taken as the border line.A treatment-free interval in both groups was of at least 4-5 weeks. After 10 months, the patients in groups A and B continued to receive similar treatments, as described for group A. A total of 92 cycles of therapy (carboplatinum + docetaxel) were given (42 in group A and 50 in group B). Some patients required dose reduction, some had a delay in starting subsequent planned cycles.
2.3. Statistical Analysis
- Analysis of survival was based on the Kaplan–Meier method [23] and events were compared using the log-rank (LR) test. The Wilcoxon test was also applied, as sometimes weighted tests give more satisfactory results than LR test [24]. Additionally, survival curves were fitted and compared by exponential lines according to the simplest equation: S=e-kt, where S is survival in relative units, t is elapsed time in years, and k is the hazard rate ( instantaneous rate of death ) in months-1. The coefficient of determination (R2) was used to determine how close the data fit these lines.Because the systemic therapy was based largely on the myelopoietic potency of patients [25], they were divided into two subgroups: those with Lph0 < 2×109 cells/L (subgroup W) and without basal lymphocytopenia (Lph0 ≥ 2×109cells/L, subgroup WO). Peripheral blood testing was performed monthly, on average, and life span was recorded for all patients every 1-2 months after the patients began therapy. All data were analyzed retrospectively. The mean numbers of lymphocytes and their standard errors (M±SE) were compared using the Student’s t-test. The variability of the data was evaluated using the coefficient of variation (CV = SD/M, where SD is the standard deviation). The relationship between LS and average numbers of lymphocytes at 10 months after treatment initiation (Lph10) was approximated by a regression line with calculated R2. The validity of the R2 value was assessed using an automatic goodness-of-fit function in Microsoft Excel. A t-test regression was used to confirm the R values [26]:
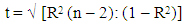 | (1) |
3. Results
3.1. Survival
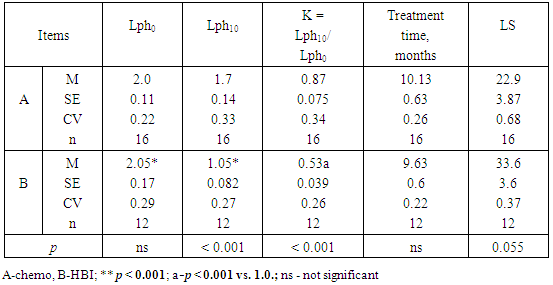
- No one of 28 patients who entered the study remains alive with follow-up of 5years. Dissemination of intraperitoneal ovarian cancer with severe ascites, adhesive peritonitis, cancer-related cardiopulmonary syndrome, intestinal and bowel obstructions and cancer-related irreversible cachexia were accounting for all deaths. The aggregate results of the replacement of four courses of conventional chemotherapy by four courses of HBI are given in Figure 1 and Table 2.
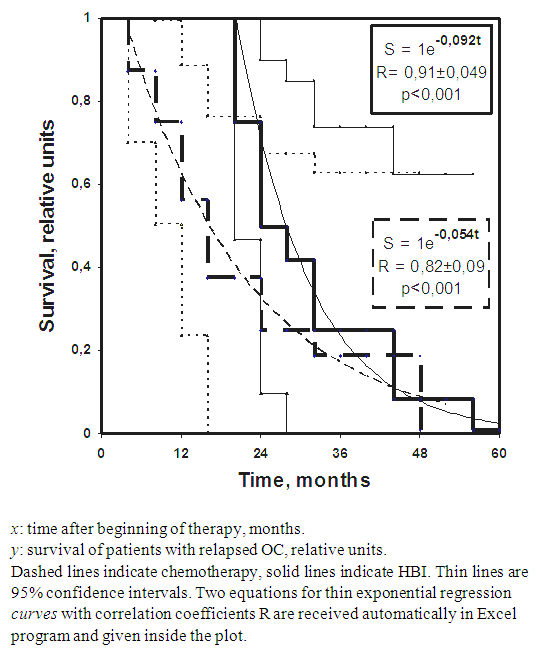 | Figure 1. Disease-specific survival of patents with relapsed OC treated with chemotherapy (thick dashed line) and HBI (thick solid line) |
|
3.2. Assessment of Relation between Cytopenia and LS
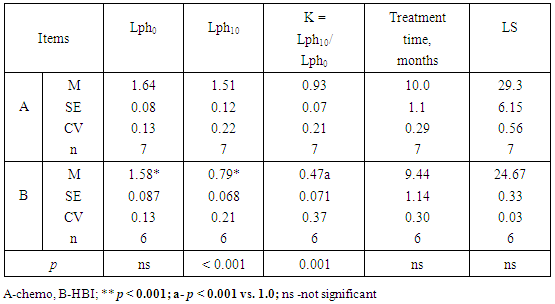
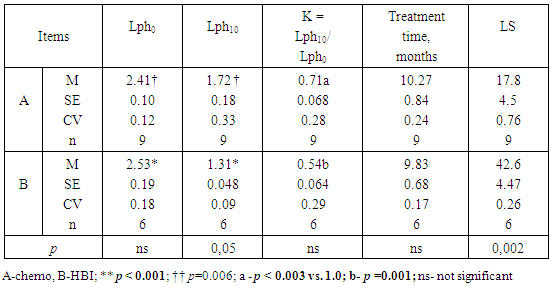
- The long-term lymphocytopenia caused by HBI seemed to be related to a longer LS (Table 2; p = 0.055). To verify this, the data in Table 2 were divided into two subgroups of patients with different baseline levels of lymphocytes (Lph0). A possible contributory role of lymphocytopenia is shown on Tables 3 and 4.
|
|
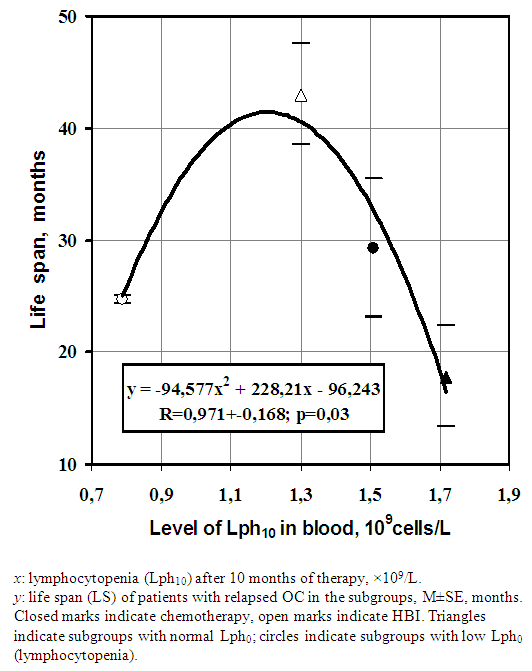 | Figure 2. Dependence of LS on long-term lymphocytopenia (Lph10) caused by different treatments |
4. Discussion
- Relatively small number of cases (n=16+12=28) is limitation of present study. However, the one case of treatment of advanced OC with HBI (0.1Gy × 15 during 5 weeks), reported by [1], as a result of immune enhancement does not seem to be relevant. Moreover, HBI in that study had been combined with local irradiation, the total dose of which in the tumor (39 Gy) exceeded 30 Gy, where 30 Gy is the typical conventional dose of local fractionated treatment according to [27]. In contrast to the results of the present study, the authors of study [1] also doubted the notion of using low-dose TBI for tumor control applications in advanced cases. Replacement of four courses of conventional chemotherapy with four courses of HBI (total dose 4 Gy) did not lead to worsened survival of advanced patients (Figure 1 and Table 2).It has been reported that deeper lymphocytopenia (≈ 0.7.109/L) in patients with primary OC reflected the optimum for therapeutic mielosuppression after 1 month [11]. The optimum for therapeutic mielosuppression found at 10 months of treatment was represented by mild lymphocytopenia (≈ (1.3-1.1) ×109/L; Figure 2). This moderate level indicates that the high repopulating capacity of the bone marrow needs to be maintained long term during cytotoxic treatment to achieve the maximal benefit [19, 28, 29]. Thus, optimum of therapeutic myelosuppression reflects low limit regenerative potential/potency of BM, below that the risk of death from the delay in renewal cells in normal tissues exceeds the risk from a malignant process as such.The following lines of evidence agree well with the notion of collaboration between host circulating cells and the tumor, compromising the existence of effective anti-neoplastic immunity in humans. The cytopenia 0.5×109 lymphocytes/L is comparable that of those who survived the nuclear bombing [30], however it is classified by [25] as a "moderate" side effect of any cytotoxic therapy. Some myelosuppression is inherent in 85% of basic anti-neoplastic drugs [31, 32]. Cancer survivors after treatment have an increased risk of developing new malignancies, by 14%, compared with the general population [33], and the anti-neoplastic agents are toxic, carcinogenic, mutagenic, clastogenic, and teratogenic with regard to normal somatic cells [34]. These few arguments of many have led to reconsideration of the treatment's principals for malignancies, which have a quasi-embryonic nature and feed by host.The remaining after therapy morphogenic potential of the BM and blood cells is directed toward the recovery of the extensive somatic damages (i.e., “side effects”) and then redirected to target the malignant tissue again only several months later [19]. Complete recovery of the initial level of blood lymphocytes can take at least 1 year, even after a single nonlethal dose of HBI [22]. Thus, so-called partial remission or even complete remission arises temporarily between the courses of cytotoxic therapy [3, 19]. This mechanism is dominant during the first phase of the tumor’s development, which is favorable for cytotoxic treatment. It may correspond to the right branch in Figure 2.The second late phase is characterized by partial exhaustion of hematopoiesis and an unstable (turbulent) regime of its self-renewal [17, 19, 28, 29]. As a result, the second phase is accompanied by a relative deficit of morphogenic cells in blood and other tissues, by relative resistance to cytotoxic therapy, and by an increased probability of chronic homeostatic imbalance between gained and lost biomass in the body, referred to as cachexia. The second phase corresponds to the left branch in Figure 2, in which the risk of overload toxicity and benefit loss is maximal. The optimum in Figure 2 reflects the equilibrium between excessive (left branch) and absent (right branch) treatment cytotoxicity. The level of induced cytopenia, which is optimal for the treatment’s benefit, reflects a competitive compromise between retardation of the cells renewing in the tumor and in normal proliferating bone marrow. In radiobiological terms, the therapeutic limit ≥ 0.5×109 lymphocytes /L [25]) is equal to ≈ 2-3 Gy of a non-lethal single dose of TBI in a healthy man [35]. The injury caused by this doses obviously insufficient for direct inactivation of tumor tissue, because even a typical palliative dose of local irradiation, such as 30 Gy in 10 fractions, has been incapable of controlling disease on a long-term basis [27, 36]. The aggregated data provide a logical background regarding the morphogenic (feeding) function of some circulating cells originating from the BM with “homing” in relation to proliferating tissues, including malignant ones [37]. The versatility of the explanation proposed in the present study is demonstrated by its applicability in the elimination of most of the basal contradictions mentioned in the Introduction and Discussion. Moreover, it helps explain the lower rate of cancer incidence in areas with higher background radiation levels [38-40], the protection against lung cancer induced by low-dose irradiation in animals [41, 42], and the discrepancies between cancer incidences and longevity among those exposed to technogenic or accidental levels of radiation [16, 43].
5. Conclusions
- Fractionated longitudinal irradiation of the lower part of the body in patients with relapsed OC contributed to longevity more, or at least not less, than did conventional chemotherapy, which was directly dependent on an optimal (healing) level of lymphocytopenia achieved 10 months after treatment initiation. These data indicate the need to balance the required level of myelosuppression with the hematopoietic resources of the patient independently of the type of systemic therapy.
ACKNOWLEDGEMENTS
- There were no special funds or grants, except regular financing of research by the Ministry of Health Care to which the Center is affiliated.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML