| [1] | AltemeierWa, Gall EA, Zinninger MM, Hoxworth PI. Sclerosing carcinoma of the major intrahepatic ducts, Arch Surg 1957;75:450. |
| [2] | Blumgart LH, Benjamin IS. Cancer of the bile ducts. In: Blumgart LH (ed.) Surgery of the Liver and biliary tract, 2ndedn. Ediburgh: Churchill Livingstone, 1994, pp967-95. |
| [3] | Anderson CD, Pinson W, Berlin J, Chari RS. Diagnosis and treatment of cholangiocarcinoma. The Oncologist 2004, 9: (1):43-57. |
| [4] | Benjamin IS. Benign and malignant lesions of the biliary tract. In: serio: Carter D, Garden JO, Paterson-Brown S (eds) Hepato-biliary and pancreatic surgery. Ed O. James Garden, W B Saunders Company ltd. 1997, pp201-258. |
| [5] | Green A, Uttaravichen T, Bhuddhisawasdi V et al. Cholangiocarcinoma in North East Thailand. A hospital - based study. Trop Geogr Med 1991;43:193-8. |
| [6] | Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the portahepatis: an unusual tumour with distinctive clinical and pathological features. Am J Med 1965; 38:241-256. |
| [7] | Ohta T, Nagakawa T, Ueno K et al. Clinical experience of biliary tract carcinoma associated with anomalous union of the pancreatic ductal system. Jpn J Surg 1990;20:36-43. |
| [8] | Olsen JH, DragstedL,Autrup H. Cancer risk and occupational exposure to aflatoxins in Denmark. Br J Cancer 1988;58(3): 392-6. |
| [9] | Bond GG, McLaren EA, Sabel FL et al. Liver and biliary tract cancer among chemical workers. Am J Ind Med 1990;18: 19-24. |
| [10] | Weinbren K, Mutum SS. Pathological aspects of cholangiocarcinoma. J Pathol 1983; 139:217-38. |
| [11] | Kato I, Kuroishi t, Tominga S. Descriptive epidemiology of subsites of cancers of the liver, biliary tract and pancreas in Japan. Jpn J ClinOncol 1989;20: 232-7. |
| [12] | Bhuiya MR, Nimura Y, Kamiya J et al. Clinico-pathological studies on perineural invasion of bile duct carcinoma. Ann Surg 1992;215; 344-9. |
| [13] | Renard P, Boutron MC, Faivre J et al. Biliary tract cancers in Cote d’Ore (France): incidence and natural history. J. Epidemiol Community Health 1987;41:344-8. |
| [14] | Davis RI, Sloan MJH, Hood JM, Maxwell P. Carcinoma of the extrahepatic biliary tree: a clinicopathological and immunohistochemical study, Histopathology 1988;12:623- 31. |
| [15] | Adam A, Benjamin IS. The staging of cholangiocarcinoma. ClinRadiol 1992;46:299-303. |
| [16] | Bisthmuth H, Castaing D, Traynor O. Resection or palliation: priority of surgery in the treatment of hilar cancer. World J Surg 1988; 12:37-47. |
| [17] | Blumgart LH, Benjamin IS. Liver resection for bile duct cancer. SurgClin North Am 1989; 69:323-37. |
| [18] | Paganuzzi M, OnettoM ,Marroni P et al. CA19-9 and CA50 in benign and malignant pancreatic and biliary disease. Cancer 1988; 61:2100-8. |
| [19] | Halliday AW, Benjamin IS, Blumgart LH. Nutritional risk factors in major hepatobiliary surgery. J Prent Ent Nutr 1988: 12:43-8. |
| [20] | Engels JT, Bafle DM, Lee JKT. Biliary carcinoma: CT evaluation of extrahepatic spread. Radiology 1989;172: 35-40. |
| [21] | Cameron JL. Proximal cholangiocarcinomas. Br J Surg 1988; 75:1155-6. |
| [22] | Desa LA, Akosa AB, Lazzara S, Domizio P, Kraus T, Benjamin IS. Cytodiagnosis in the management of extrahepatic biliary stricture. Gut 1991;32:1188-91. |
| [23] | Garden OJ. Intraoperative and laparoscopic ultrasonograpy. Oxford: Blackwell Science,1995. |
| [24] | Nakeeb A, Tran KQ, Black MJ, et al. Improved survival in resected biliary malignancies. Surgery2002:132:555-564. |
| [25] | Abdulla EK, Barnett CC, Doherty D et al. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolisation. Arch Surg 2002; 137; 675-681. |
| [26] | Nimura Y, Hayakawa n, Kamiya J, Kondo S, Shiyonoya S. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg 1990; 14:535-44. |
| [27] | Baer HU, Stain SC, Dennison AR, Egers B. Blumgart LH. Improvements in survival by aggressive resections of hilarcholangiocarcinoma. Ann Surg 1993;27: 20-7. |
| [28] | Guthrie CM, Haddock G, de Beaux AC, Garden OJ, Carter DC. Changing trends in the management of extrahepatic cholangiocarcinoma. Br J Surg 1993;80:1434-9. |
| [29] | Baton O, Azoulay D, Adam DV, et al. Major hepatectomy for hilarcholangiocarcinoma type 3 and 4:Prognostic factors and long term outcomes, J. Am CollSurg 2007;204:250-260. |
| [30] | Pinson CW, Rossi RL. Extended right hepatic lobectomy, left hepatic lobectomy and skeletonization resection for proximal bile duct cancer. World J Surg 1988;12:52-59. |
| [31] | Tsao JL Nimura Y, Kamiya T et al. Management of hilarcholangiocarcinoma: a comparison of an American and Japanese experience. Ann Surg 2000;232:166-174. |
| [32] | Hasegawa S, Ikai I, Fujii H, et al. Surgical resection of hilarcholangiocarcinoma: analysis of survival and post operative complications. World J Surg 2007; 31: 1256-1263. |
| [33] | Lillemore KD, Cameron JL. Surgery forhilarcholangiocarcinma: the Johns Hopkins approach. J. Hepatobiliary Pancreat Surg 2000;7:155-162. |
| [34] | Saldinger PF, Blumgart LH. Resection of hilar cholangiocarcinoma- a European and United states experience. J. HepatobiliaryPancreat Surg.2000;7:111-114. |
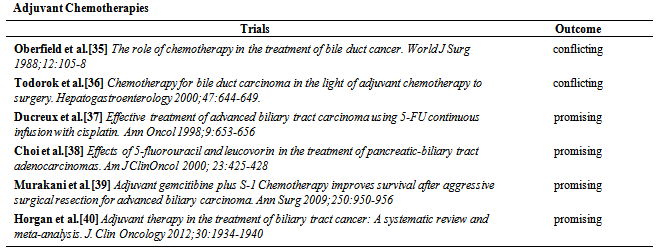
| [35] | Oberfield RA, Rossi RL. The role of chemotherapy in the treatment of bile duct cancer. World J Surg 1988;12:105-8. |
| [36] | Todorok T. Chemotherapy for bile duct carcinoma in the light of adjuvant chemotherapy to surgery.Hepatogastroenterology 2000;47:644-649. |
| [37] | Murakani Y, Uemura K, Sudo et al. Adjuvant gemcitibine plus S-1 Chemotherapy improves survival after aggressive surgical resection for advanced biliary carcinoma. Ann Surg 2009;250:950-956. |
| [38] | Choi CW, Choi IK, Seo TH et al. Effects of 5-fluorouracil and leucovorin in the treatment of pancreatic-biliary tract adenocarcinomas. Am J ClinOncol 2000; 23:425-428. |
| [39] | Ducreux M, Rougier P, Fand A et al. Effective treatment of advanced biliary tract carcinoma using 5-FU continuous infusion with cisplatin. Ann Oncol 1998;9:653-656 . |
| [40] | Horgan AM, Amir E, Walter T, Knox JF. Adjuvant therapy in the treatment of biliary tract cancer: A systematic review and meta-analysis. J. Clin Oncology 2012;30:1934-1940. |
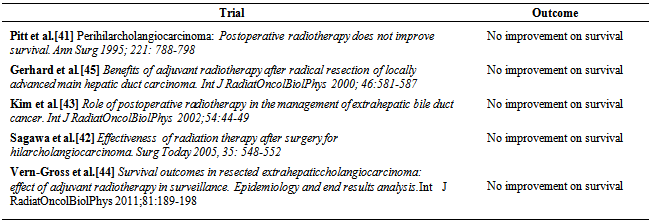
| [41] | Pitt HA, Nakeeb A, Abrams RA et al. Perihilar cholangiocarcinoma: Post operative radiotherapy does not improve survival. Ann Surg 1995; 221: 788-798. |
| [42] | Sagawa N, Kondo S, Morikawa T et al. Effectiveness of radiation therapy after surgery for hilarcholangiocarcinoma. Surg Today 2005, 35: 548-552. |
| [43] | Kim S, Kim SW, Bang Y J et al. Role of postoperative radiotherapy in the management of extrahepatic bile duct cancer. Int J RadiatOncolBiolPhys 2002;54:44-49. |
| [44] | Vern-Gross TZ, Shivnani AT, Chan K et al. Survival outcomes in resected extrahepaticcholangiocarcinoma: effect of adjuvant radiotherapy in surveillance. Epidemiology and end results analysis. Int J RadiatOncolBiolPhys 2011;81: 189-198. |
| [45] | Gerhards MF, van Gulik TM, Gonzalez D et al. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J. surg 2003;27:173-179. |
| [46] | Todorok T, O’hara K, Kawannut T et al. Benefits of adjuvant radiotherapy after radical resection of locally advanced main hepatic duct carcinoma. Int J Radiat Oncol Biol Phys 2000; 46:581-587. |
| [47] | Heron DE, Stein DE, Eschelman CJ et al. Cholangiocarcinoma: The impact of tumour location and treatment strategy on outcome. Am J Clin Oncol 2003; 26:422-428. |
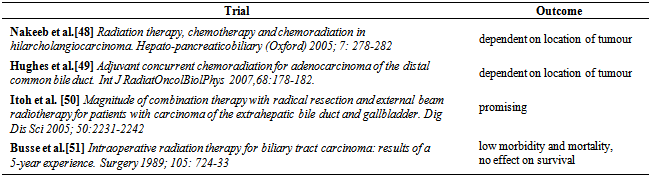
| [48] | Nakeeb A, Pitt HA. Radiation therapy, chemo therapy and chemoradiation in hilar cholangiocarcinoma. Hepato- pancreaticobiliary (Oxford) 2005; 7: 278-282. |
| [49] | Hughes Ma, Trassica AA, Yeo CJ et al. Adjuvant concurrent chemoradiation for adenocarcinoma of the distal common bile duct. Int J RadiatOncolBiolPhys 2007,68:178-182. |
| [50] | Itoh H, Nishijimak, Kurosaka Y et al. Magnitude of combination therapy with radical resection and external beam radiotherapy for patients with carcinoma of the extrahepatic bile duct and gallbladder. Dig Dis Sci 2005; 50:2231-2242. |
| [51] | Busse PM, Stone MD, Sheldon TA et al. Intraoperative radiation therapy for biliary tract carcinoma: results of a 5-year experience. Surgery 1989; 105: 724-33. |
| [52] | Hudd C, LaRegina MC, Devine JE et al. Response to exogenous cholecystokinin of six human gastrointestinal cancers xenografted in nude mice. Am J Surg 1989;29: A736-7. |
| [53] | Jeyarajah DR, Klintmalm GB. Is liver transplantation indicated for cholangiocarcinoma? J Hepatobiliary Pancretsurg 1998;5:48-51. |
| [54] | Pichlmayr R, Burckhardt R, Lauchert W, Beckstein WO, Gubernatis G, Wagner E.. Radical resection and liver grafting as the two main components of surgical strategy in the treatment of proximal bile duct cancer. World J Surg 1988;12:68-77. |
| [55] | De Vreede I, Steers JL, Burch PA et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoradiation for cholangiocarcinoma. Am J. Transplant 2002;2:774-779. |
| [56] | Meyer CG, Penn I, James L. Liver transplantation for cholangiocarcinoma: results in 207 patients. Transplantation 2000;69:1633-1637. |
| [57] | Hatfield ARW. Palliation of malignant obstructive jaundice- surgery or stent? Gut 1990;31:1339-40. |
| [58] | Cheng JL, Bruno MJ, Bergman JJ. Endoscopic palliation of patients with biliary obstruction caused by non-resectable hilar cholangiocarcinoma: efficacy of self-expandible metallic wall stents. GastrointestEndos 2002, 56:33-39. |
| [59] | Casola G, O’Laoide R. Hilar cholangiocarcinoma: percutanous approach. In:Serio G, Huguet C, Williamson RCN (eds) Hepato-biliary and pancreatic tumours. Edinburgh: Graffham Press,1994, pp123-6. |
| [60] | Terblanche J, Saunders SJ, Louw JH. Prolonged palliation in carcinoma of the main hepatic duct junction. Surgery 1972;71:720-31. |
| [61] | Guthrie CM, Banting SW, Garden OJ, Carter DC. Segment III cholangiojejunostomy for palliation of malignant hilar obstruction. Br J Surg 1994;81: 1639-41. |
| [62] | Wiedmann M, Caca K, Berr F et al. Neoadjuvant photodynamic therapy – a new approach to treating hilarcholangiocarcinoma: a phase II pilot study. Cancer 2002; 97:2783-2790. |
| [63] | Vallis KA, Benjamin IS, Munro AJ et al. External beam and intraluminal radiotherapy for locally advanced bile duct cancer: role and tolerability. RadiotherOncol 1996. |
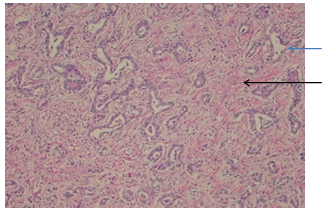
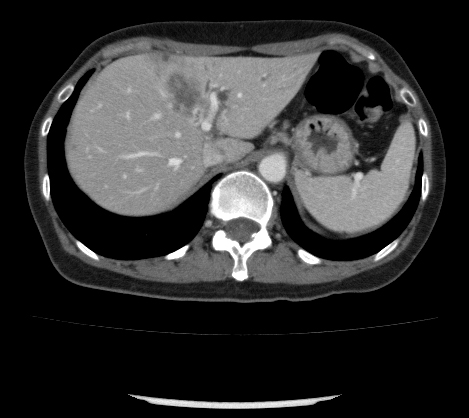
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML