-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Tumor Therapy
p-ISSN: 2163-2189 e-ISSN: 2163-2197
2013; 2(1): 26-34
doi:10.5923/j.ijtt.20130201.04

Comparison between CEA, CA 19-9 and CA 72-4 in Patients with Colon Cancer
Eman M. I. Youssef1, Gehan H. Ewieda1, Haneya A. A. Ali2, Amany M. Tawfik2, Wafaa Mohi El-deen Abd El-fatah1, Amgad A. Ezzat3, 4, Rehab M. Elsaid Tash5, Nashwa El-Khouly6
1¹Department of Medical Biochemistry, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
2²Department of Microbiology & Immunology, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
3³Department of Microbiology & Immunology, Faculty of Medicine, Al-Azhar University, Assuit, Egypt
4Department of Microbiology & Immunology, Faculty of Medicine, Tabuk University, KSA
5Department of Microbiology & Immunology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
6Department of Internal Medicine, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt
Correspondence to: Eman M. I. Youssef, ¹Department of Medical Biochemistry, Faculty of Medicine (for girls), Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2013 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background:In Egypt, colorectal cancer (CRC) is one of the most common malignancies and represents 6.5% of cancers. The most important currently available tumor markers in CRC that provide diagnostic information to reduce mortality and morbidity are carcinoembryonic-antigen (CEA), carbohydrate antigen 19-9 (CA19-9) and CA 72-4. The main objectives of the present work were to compare the serological tumor markers CEA, CA19-9 and CA 72-4 and to verify the effectiveness of each marker as a potential routine diagnostic test in CRC. Subjects and methods:Blood samples were collected from twenty five CRC patients then compared with thirty five normal blood samples from apparently healthy persons who were considered as controls. Serum of each sample was evaluated for the three tumor markers and assessed by ELISA technique Results:CA72-4 and CEA showed high statistically significant difference between CRC and controls, on the contrary CA19-9 showed a statistically insignificant between the studied groups. As for the results of the ROC curve, the sensitivity for using CA 72-4 parameter was 82.86%, the specificity was 100% and the highest AUC for CA72-4 denoting its performance as the preferred diagnostic routinetest among other markers mentioned above. Conclusions:Based on our findings, the results of this study indicated that the serum CEA is not a much more sensitive tumor marker than CA72-4. As well as the serum CA72-4 levels can be used in diagnosis of colon cancer and in need to be applied as a routine tumor marker.
Keywords: Colorectal cancer (CRC), Carcinoembryonic antigen (CEA),Cancer antigen 19-9 (CA 19-9), Cancer antigen 72-4 (CA72-4), Area undercurve (AUC)
Cite this paper: Eman M. I. Youssef, Gehan H. Ewieda, Haneya A. A. Ali, Amany M. Tawfik, Wafaa Mohi El-deen Abd El-fatah, Amgad A. Ezzat, Rehab M. Elsaid Tash, Nashwa El-Khouly, Comparison between CEA, CA 19-9 and CA 72-4 in Patients with Colon Cancer, International Journal of Tumor Therapy, Vol. 2 No. 1, 2013, pp. 26-34. doi: 10.5923/j.ijtt.20130201.04.
Article Outline
1. Introduction
- Colorectal cancer (CRC) is the third most common cancer worldwide after lung and breast cancers and the third leading cause of cancer-related death in both men and women in industrialized countries [1,2]. The genesis of CRC involves series of steps in which environmental and/or endogenous carcinogens induce or promote cancer development [3]. The development of CRC from normal epithelial cells through benign adenomas to malignant carcinomas and metastasis is multisteps process [4, 5], involving accumulation of mutations of key regulatory genes [4]. These steps include the activation of oncogenesand inactivation of tumor suppressor genes and genes involved in DNA mismatch repair [6,7]. In clinical practice, tumor markers are expressed by tumor tissue and potentially useful in screening for cancer, monitoring the course of the disease and detecting the recurrence after the treatment due to higher levels have been observed in advanced disease [8-10]. Unfortunately, no tumor marker with high specificity and sensitivity could become a routine diagnostic tool for CRC and the diagnosis is often made at too late stage of the disease when curative treatment is not possible and this induces a poor prognosis [11-13].CEA is a single chain glycoprotein containing 30 to 70 weight percent of carbohydrates. It is expressed in significant amounts during embryonic life, especially by the large intestine, and postnatally by carcinomas arising from this site [14, 15]. CEA level is elevated in many malignancies such as digestivetract cancers, breast cancer, lung cancer, metastatic diseases of the liver, pancreatic carcinoma and medullary carcinoma of the thyroid. It can be released by these tumours into the circulation to cause raised levels [16, 17]. Small amounts of CEA are also present in the normal adult large intestine and in the circulation of healthy subjects. However, CEA level is also increased innon-malignant disorders, for examples, liver diseases, active inflammatory bowel disease, and aging. Heavy cigarettesmokers have higher serum CEA than healthy non-smokers [18].The CA19-9 is synthesized by normal human pancreatic and biliary cells, gastric and colonic epithelial cells as well as in large quantities in normal pancreatic juice. It is expressed on tumour cells and plays a role in adhesion between tumour cells and endothelial cells.It
 is secreted into the serum and is used as a tumour marker for pancreatic, hepatobiliary, gynecological, and CRC [19, 20]. Elevated serum levels of subjects can indicate progressive malignant disease, poor therapeutic response and recurrence before being detected by radiographs or clinical findings. Adeclining CA19-9 value may be indicative of a favorable prognosis and good response to treatment [21-23]. CA72-4 is synthesized and expressed by both normal and malignant cells of the gastrointestinal tract. Elevated CA72-4 levels in serum and plasma have been reported in various malignant diseases including carcinoma of colon, stomach, pancreas and gall bladder [15, 24]. Also, elevated serum values can be found in benign disorders such as: pancreatitis, cirrhosis of the liver, pulmonary diseases, rheumatic illnesses, gynecological illnesses, benign diseases of the ovaries, ovarian cysts, as well as diseases of the breast [14, 25].
is secreted into the serum and is used as a tumour marker for pancreatic, hepatobiliary, gynecological, and CRC [19, 20]. Elevated serum levels of subjects can indicate progressive malignant disease, poor therapeutic response and recurrence before being detected by radiographs or clinical findings. Adeclining CA19-9 value may be indicative of a favorable prognosis and good response to treatment [21-23]. CA72-4 is synthesized and expressed by both normal and malignant cells of the gastrointestinal tract. Elevated CA72-4 levels in serum and plasma have been reported in various malignant diseases including carcinoma of colon, stomach, pancreas and gall bladder [15, 24]. Also, elevated serum values can be found in benign disorders such as: pancreatitis, cirrhosis of the liver, pulmonary diseases, rheumatic illnesses, gynecological illnesses, benign diseases of the ovaries, ovarian cysts, as well as diseases of the breast [14, 25].2. Subjects and Methods
2.1. Subjects
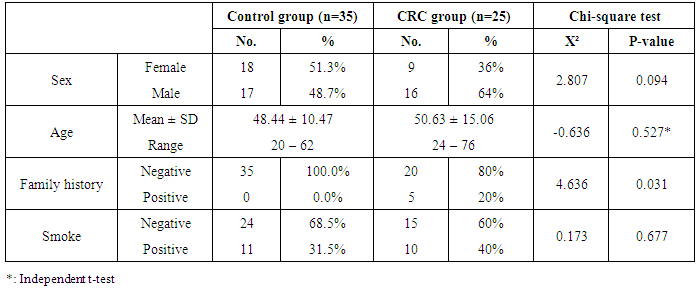
- This study was carried out on 60 subjects in the Department of Internal Medicine, Faculty of Medicine, Al-Zaharaa University Hospital, Cairo, Egypt. Our study was case control study including patients attending to Al-Zaharaa University Hospital as in the outpatient clinic in a period of 9 months during 2012. The included subjects in this study were divided into two groups: Group (I) included thirty five healthy control subjects without any evidence of any disease, 17 males (48.7%) and 18 females (51.3%), their ages were between 20-62 years. Group (II) included twenty five CRC patients, with no other cancer, 16 males (64%) and 9 females (36%), their ages were between 24-76 years. Both groups were age and gender-matched. Ethical clearance: Informed oral consents were taken from all participants in this study.
2.2. Methods
- CRC patients and controls included in the study were subjected to the following: Full history taking and complete clinical examinations. Radiological investigations include: Abdominal ultrasound and CT, chest X – ray and lower gastrointestinal endoscopy (colonoscopy) and biopsy taking of colorectal cancer tissue for histopathological examinations to confirm the diagnosis. Specific laboratory investigations including tumor markers: CEA, CA 19-9 and CA 72-4.
2.3. Detection of Tumor Markers in Human Serum
- Blood samples were collected in 5 ml disposable syringe. Serum was separated from the blood by allowing it to complete clot and centrifuged at 3000 rpm for 10 minutes. The serum of each sample was divided into 3 portions. Specimens should be capped and stored at -20°C until analysis time. Avoid repeated freezing and thawing of serum samples. Serum of each sample was evaluated for the above mentioned tumor markers. The CEA and CA19-9 enzyme immunoassay test kits were supplied from Immunospec Corporation, 7018 Owensmouth Ave, Suite 103, Canoga Park, CA, 91303and CA 72-4 ELISA kit was supplied from DRG International, Inc., USA.
2.3.1. Quantitative Measurement of CEA
- The Immunospec CEA is a quantitative solid phase enzyme linkedimmunsorbent assay (ELISA), (Catalog No.E1-207). The wells are coated with anti-CEA antibodies. The samples, standards and controls are incubated in the wells with enzyme conjugate which is anotherantibody directed toward a different region of CEA molecules and chemically conjugated with horseradish peroxidase. Unboundenzyme conjugate is washed off and the amount of boundperoxidase is proportional to the concentration of the CEA present in the samples, standards and controls. Upon addition of the TMB substrate, the intensity of color developed isproportional to the concentration of CEA in the serum. Theoptical density of the colored samples is read with a microplatereader at 450 nm.
2.3.2. Quantitative Measurement of CA19-9
- Immunospec CA19-9 EIA test is a solid phase two-siteimmunoassay, (Catalog No. E29-210). One monoclonal antibody is coated on the surface of the microtiter wells and another monoclonal antibody labeled with horseradish peroxidase is used as the tracer. The CA19-9molecules present in the standard solution or serum are "sandwiched" between the two antibodies. Following the formation of the coated antibody-antigen-antibody-enzyme complex, the unbound antibody-enzyme labels are removed by washing.
2.3.3. Quantitative Measurement of CA 72-4
- The DRG TM-CA 72-4 (Catalog No. EIA-5071) ELISA Kit is a solid phase enzyme-linked immunosorbent assay (ELISA) based on the sandwich. The microtiter wells are coated with a monoclonal mouse antibody (Clone CC49) directed towards a unique antigenic site on a CA 72-4 molecule. An aliquot of sample containing endogenous CA 72-4 is incubated in the coated well with enzyme conjugate, which is an anti-CA 72-4 antibody (Clone B72.3) conjugated with horseradish peroxidase. After incubation the unbound conjugate is washed off.
2.4. Statistical Analysis
- Statistical analyses were performed with SPSS 20 software. Parametric data were summarized using mean ±SD, whereas nonparametric data were summarized as median and percentiles for quantitative variables, and frequency and percentages were used for qualitative variables. Comparison between groups was done using the Chisquare test and non-parametric Mann-Whitney U test were used to compare two groups. The Receiver Operating Characteristic (ROC) curve was used for prediction of cut off values. P values less than 0.05 were considered of significance.
3. Results
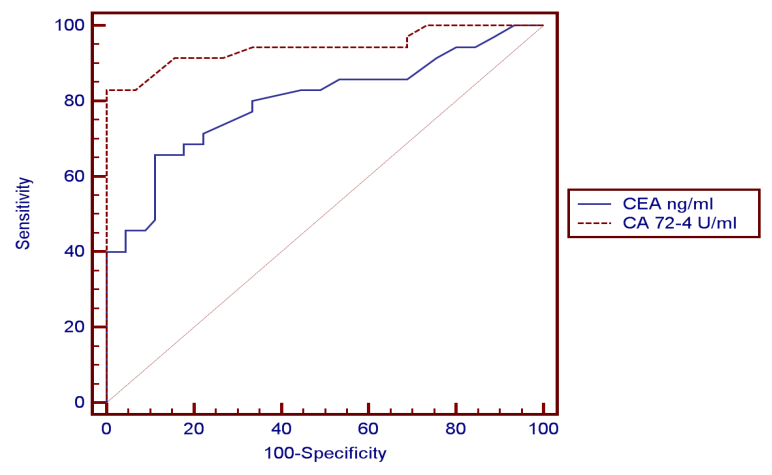
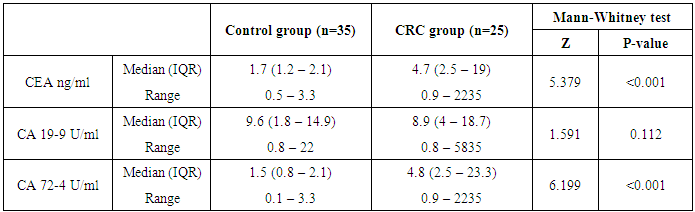
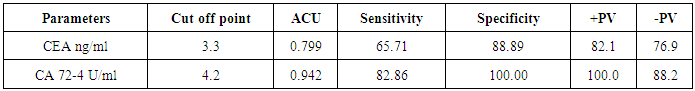
- The present study demonstrated that the mean age value for 25 studied CRC patients was 50.63 years with more than 1/3 of patients (31.5%) are ≤ 40 years and those above 60 represent 35.8%. The mean age for controls was 48.44 years. Ten patients 10/25 (40%) and 11/35 (31.5%) healthy controls were smokers. No significant difference in smoking could be detected between both groups (P=0.677).Only 5/25 (20%) patients gave positive family history of CRC while none of the control subjects gave positive family history, this difference between the two groups was statistically significant (P=0.03).On comparing the medians of the three tumor markers (CEA, CA19-9 and CA72-4) between control subjects and cases, it was found that among the cases, the median of CEA was 4.7 ng/ml, CA19-9 was 8.9U/ml and CA72-4 was 4.8 U/ml and among the controls, the medians of the three tumor markers were1.7 ng/ml, 9.6 U/ml and 1.5 U/ml respectively. CA72-4 and CEA showed a high statistically significant difference between both groups. On the other hand, CA19-9 showed statistically insignificant difference between two groups.In the current study a ROC curve was constructed for comparing the two statistically significant tumor markers (CEA and CA72-4) in the diagnosis of CRC as for their sensitivity, specificity and the AUC for the two markers were 0.799 and 0.942 respectively. By considering the cut-off values at 3.3ng/ml for CEA and at 4.2 U/ml for CA72-4, the sensitivity and specificity for CEA were 65.71% and 88.89% respectively. Finally, the sensitivity and specificity for CA72-4 were 82.86% and 100% respectively. The following figure (3) shows plotted receiver operating characteristic (ROC) curves of CEA and CA72-4 statistically significant tumor markers to detect the best cut off values for these markers.
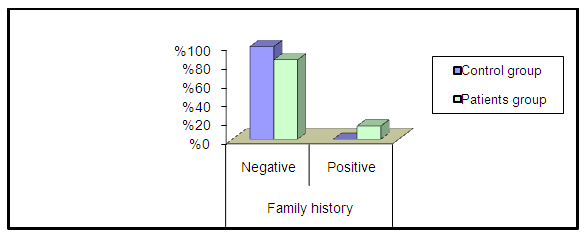 | Figure 1. Percentage of family history among two groups |
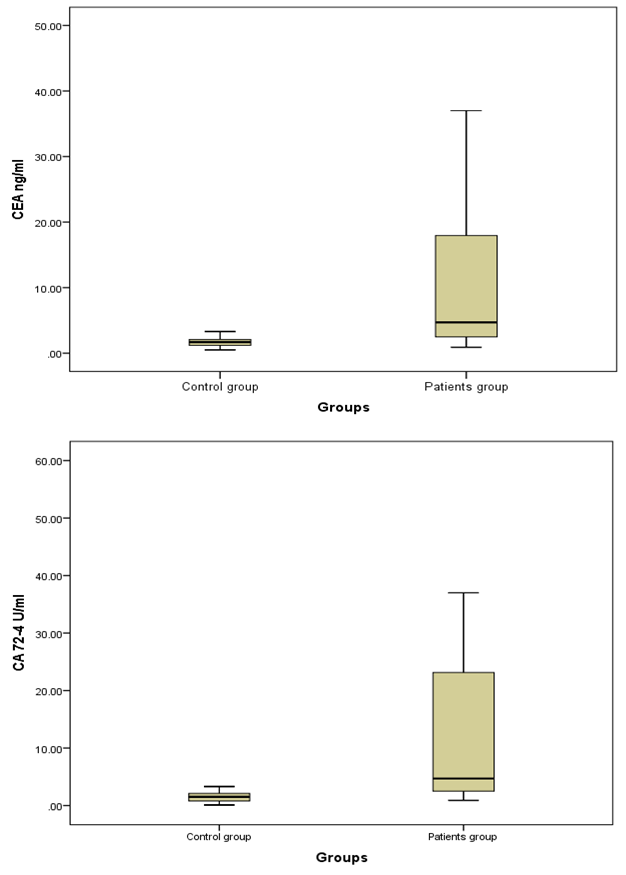 | Figure 2. Box plots for value distribution of CEA ng/ml and CA72-4 U/ml levels in the two groups; Bars show the medians and boxes the interquartile range |
|
|
|
4. Discussion
- CRC is a multifactorial disease with dietary, lifestyle and environmental exposures on one hand and genetic predispositions on the other hand. The risk of CRC begins to increase above the age of 40 years and rises sharply at ages 50 to 55 years [26]. In our study, the mean age of CRC patients was 50.63 years. Maximum incidence was between 24 and 76 years, 31.5% were less than 40 and those above 60 represent 35.8%. In agreement with our results, El Bolkieny et al. [27] in a series of 215 patients reported that the mean age of CRC patients in Egypt is 51 years. A slightly higher age mean (55 years) among Egyptian CRC patients was reported by Ibrahim et al. [28]. However in western countries CRC is considered the disease of elder population. Max et al. [29] reported in his study on global cancer statistics that in the west the mean age of CRC patients is about 65 years. The average age to develop CRC is 70 years, and 93% of cases occur in persons 50 years of age or older Rex et al. [30].The age distribution in our study is comparable to those reported by other researchers on Egyptian patients such as Abo Zaid et al. [31] and Afify et al. [32] who reported that CRC in Egypt has a unique characteristics that differ from that reported in other countries of the western society where most of the cases of CRC are elderly and that CRC has no age predilection. Abo Zaid et al. [31] observed that more than one third of CRC tumors in Egypt affect young population. Soliman et al. [33]reported in their study that 44% of the patient series was under the age of 40.In Egyptian patients the high prevalence of CRC in young people can neither be explained on hereditary basis nor can it be attributed to bilharziasis however can be attributed to ongoing westernization of Egypt as the country develops and affecting young people first because they are more likely to change lifestyle than older people or reflect the presence of clinically in apparent inherited syndromes Soliman et al. [33]. In the present study, the majority of CRC cases were men (64%) while women represent 36%, which comes in agreement with Richardson et al. [34] who reported in CRC, male incidence rates, adjusted for age and race, were higher than that for females. Jemal et al. [35] reported that colorectal cancer is the fourth most common cancer in men and the third most common cancer in women worldwide and exceeded the peak incidence rates observed among males. CRC incidence rates in males increased in high-income countries such as the United States. Hara et al. [36] also claimed that men has 20% more incidence of developing colorectal cancer than females. On the other hand these results were in contrast to those reported by Parkin et al. [37] who showed in a clinical study that women have the same risk of CRC as men. Cancer colon affects men and women almost equally. No significant predominance in either sex groups. Rex et al. [30], who documented that men tend to get colorectal cancer at an earlier age than women, but women, live longer and thus the total number of cases in men and women is equal.Tobacco smoke is a major source of a wide variety of carcinogens including heterocyclic amines, polycyclic hydrocarbons, and nitrosamines. Several studies had noted a higher risk for colon cancer among cigarette smokers, especially among those with very long smoking histories Knekt et al. [38], Giovannucci [39] andCross and Sinha [40], butthese studies were in discordance with our findings. The results of the present study showedten patients (40%) and eleven (31.5%) healthy controls were smokers. No significant difference in smoking could be detected between the two groups; p=0.677. Similar results have been previously reported by Slattery et al. [41] reported that cigarette smoking had not been often associated with an elevated risk of colorectal cancer. Our results demonstrated that there was an only five patients (20%) gave positive family history of CRC while none of the control subjects gave positive family history, this difference between the two groups was statistically significant p=0.031. As stated by Johnsand Houlston [42], some individuals are more prone to CRC than others and about 25% of colon cancer patients have some degree of familial background, and another 15% have a strong family history involving first or second degree relative.In view of the previous facts in this study, the serum CEA showed the following results, median levels of CEA were significantly higher in CRC patients when compared to its median level in control group (P<0.001).The same results were reported by Zhao et al [43]where the serum levels of CEA were determined by ELISA before surgery in 134 patients with colorectal cancer and in 200 healthy people as controls. CEA levels in patients were significantly higher than those in controls (P<0.01). The results of the present study were also in agreement to those reported by Grotowski et al [44] who found that serological expression of CEA was elevated in 50.6% of the patients with CRC at time of diagnosis while in the group of persons with inflammatory bowel diseases CEA concentration was elevated in only 2.7% of the patients. In the study conducted by Guadagni et al [45]where serum CEA, antigen levels were determined with a radioimmunometric assay in malignant (n = 200) and benign (n = 100) colorectal disease, it was shown that of the 200 patients with colorectal carcinoma, the percentage of patients whose serum samples were positive for CEA was 43%. Concerning the results of CA19-9, it was shown that statistically insignificant difference between malignant and control groups (P=0.112).These results agreed with the study done by Cerda et al. [46] which was conducted on seventy CRC patients and 32 healthy controls, where the serum levels of CA19-9 was measured. Their study showed no significant difference in CA 19-9 values between the studied groups. The same findings were reported byMorita et al. [47] could not find clinical significance to support the use of CA19-9 to predict the prognosis and detect recurrence of colorectal cancer. However Wanget al. [48] conducted a clinical study on 101 patients with colorectal adenocarcinoma, and 40 normal healthy controls, where the concentration of serum CA19-9 was determined by radioimmunoassay. Of the normal sera, only 2.5% of cases were elevated. In patients' sera, the mean value of CA19-9 levels was significantly higher in patients (P < 0.001) than in the normal healthy control. In addition the levels of CA19-9 were elevated in 50% of patients with advanced CRC while the elevation was only 18.6%of patients with localized CRC. The third serum marker in this study, CA72-4 is currently the tumor marker in use for colon cancer diagnosis. Median level of CA72-4 was significantly higher in CRC patients when compared to control group (p<0.001). The results of the present study were comparable to those reported by Yasasever et al. [49] where the values related to healthy individuals were of median 1.7 U/ml, whereas median of 12.1 U/ml was measured in malignancy with a statistically significant difference between both groups. These results also comes in harmony with Chao et al. [50] who found that colorectal cancer patients with positive serum CA72-4 had higher recurrence rate than those with negative CA72-4 and the 3-year survival rate much lower than that in CA72-4-negative patients. High serum levels of tumor marker are often associated with more aggressive cancer, which usually has short disease-free interval and short survival period. In the study conducted by Ohuchi et al. [51]where serum levels of CA72-4 antigen in patients with colorectal diseases were measured using radioimmunoassay (RIA), elevated levels of serum CA72-4 antigen were found in 67% of patients with colorectal carcinoma, while elevated serum levels of CA72-4 were found in only 7% of patients with benign disease. The difference in sensitivity between this study and the present study may be due to different epitopes used in the immunoassay. Guadagniet al. [52] conducted a study where colorectal tissue biopsies were obtained from 110 patients diagnosed as primary colorectal carcinoma (tumor and normal colonic mucosa samples), 20 patients diagnosed as benign colorectal disease, and 31 healthy donors. The level of expression of tumor-associated glycoprotein 72 (TAG-72) was quantitatively measured using RIA, a significant approximately 10-fold increase of TAG-72 expression in the colon tumor biopsies when compared with the expression in normal colonic mucosa from the same patients was observed.Concerning the results of ROC curve for CEA and CA 72-4 were shown to have the AUC = 0.799ng/ml and 0.942U/ml respectively, it showed a sensitivity of 65.71%, and specificity of 88.89% for CEA and sensitivity of 82.86%, and specificity of 100% for CA 72-4. Best diagnostic test was CA 72-4.These results were different from that found by Lopez et al. [53], who reported in a clinical study that the sensitivity of CA 72-4 in CRC patients was 56% and specificity was 100% which is comparable to the results of this study. The results of the present study also coincide with the study conducted by Carpelan-Holmstrom et al. [54]; they evaluated and compared serum tumor markers; CEA, CA 19-9 and CA 72-4, and their value in the diagnosis of malignant colorectal disease. The serum concentrations of the markers were measured in 204 patients with colorectal cancer and in 104 in patients with benign colorectal disease. Only CEA and CA 72-4 provided significant diagnostic information (p < 0.001). The probability of cancer for each patient was calculated entering CEA and CA 72-4 in the logistic regression model. Their study concluded that the diagnostic value of CA 72-4 was additive to that of CEA in colorectal cancer and both markers contributed with significant diagnostic information. Also Fernandez – Fernandez et al. [55] evaluated the usefulness of CA 72-4 tumor-associated antigen in colorectal carcinoma, they studied 70 patients with benign colorectal diseases and 127 patients with colorectal cancer at different stages. The results were compared with those obtained by CEA and CA 19-9. In their ROC curve, at a specificity of 95%, the sensitivities of CEA, CA 19-9 and CA 72-4 were 46.4%, 20.5% and 40.1%, respectively. They concluded that CA 72-4 showed better sensitivity and specificity scores than CA 19-9. CEA sensitivity was increased from 46.4% to 59.8% when combined with CA 72-4, while showing a decrease of 0.9% in specificity.
5. Conclusions
- In conclusion, the results of the current study and the corresponding studies, CEA is lower in its diagnostic significance as compared to CA 72-4. Also, CA 72-4 has the best overall sensitivity, specificity and is superior to CEA in malignant cases, so the use of CA72-4 could be considered as the most useful routine marker for diagnosis of CRC. A currently used marker CA19-9 was shown in this study to have no diagnostic value for CRC. Large number of cases is recommended using greater number of subjects to avoid statistical interference and bias, long-term follow up of these tumor markers to monitor patients receiving therapy for early prediction of recurrence after surgical resection and to assess treatment efficacy and finally in the future, more sensitive techniques using other tumor markers should be developed.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML