-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Tumor Therapy
p-ISSN: 2163-2189 e-ISSN: 2163-2197
2013; 2(1): 18-25
doi:10.5923/j.ijtt.20130201.03
The Role of Radiation in Multimodality Therapy for Esophageal Cancer
Yongshun Chen
Department of Radiation Oncology, Zhengzhou University Affiliated Cancer Hospital, Henan Cancer Hospital, Zhengzhou University, 127, Dongming Road, Zhengzhou, 450008, China
Correspondence to: Yongshun Chen , Department of Radiation Oncology, Zhengzhou University Affiliated Cancer Hospital, Henan Cancer Hospital, Zhengzhou University, 127, Dongming Road, Zhengzhou, 450008, China.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Esophageal cancer is one of the commonest malignancies with an increasing incidence, surgery is view as the standard of care, but satisfied survival figure is noted only in very early stage disease. For most of the patients presenting with advanced disease, curative treatment remains to be a challenge. Radiotherapy as curative and palliative approaches for esophageal cancer is widely used, and improved local control and survival were achieved by adding chemotherapy. Multimodality therapy including surgery, radiotherapy, chemotherapy and targeted therapy is increasingly considered a promising strategy, an evidence-based review of the role of radiation in the treatment of esophageal cancer is hereby presented.
Keywords: Esophageal Cancer, Radiation, Multimodality therapy, Prognosis
Cite this paper: Yongshun Chen , The Role of Radiation in Multimodality Therapy for Esophageal Cancer, International Journal of Tumor Therapy, Vol. 2 No. 1, 2013, pp. 18-25. doi: 10.5923/j.ijtt.20130201.03.
Article Outline
1. Introduction
- Esophageal cancer is among the tenth commonest cancers in the world and a highly virulent malignancy with average 5-year survival rates not exceeding 25%[1]. The incidence of esophageal cancer has been increasing over the past three decades, squamous cell carcinoma and adenocarcinoma account for more than 90% of all esophageal cancer cases. The incidence of squamous cell carcinoma has declined, but the incidence of esophageal adenocarcinoma has risen as a result of increases in obesity and gastroesophageal reflux disease.Esophageal cancer is often asymptomatic or causes only nonspecific symptoms in its early stages, and unfortunately barium swallow radiograph or endoscopy is not involved in periodic medical examination project in many developing countries. Patients with esophageal cancer has often reached an advanced stage by the time symptoms such as dysphagia and odynophagia start manifesting themselves, 50-60% of them presents with stage IIB-IV diseases at the time of diagnosis[2].Surgery is traditionally considered the standard treatment for esophageal cancer, but surgical resection is finally possible in just 15–20% of patients. Even for for most localized esophageal cancers, cure rates after surgery alone have been poor, with 3- to 5-year survival rates ranging from 6% to 35%[3]. The management of locally advanced esophageal or gastroesophageal junction cancer has shifted from surgery or radiation alone to multimodality approach. Radiotherapy as a curative approach for esophageal cancer is widely used, however, the sequence of treatment steps in combination with surgery and chemotherapy for different stages of this disease has not been well illustrated. To determine the optimum therapeutic strategy for this virulent cancer, we review data on surgery, radiation, chemoradiation, trimodality therapy and targeted therapy. We also examine emerging radiation technologies such as intensity-modulated radiotherapy, image-guided radiotherapy and molecular imaging in esophageal cancer management.
2. Surgery versus Definitive Radiotherapy
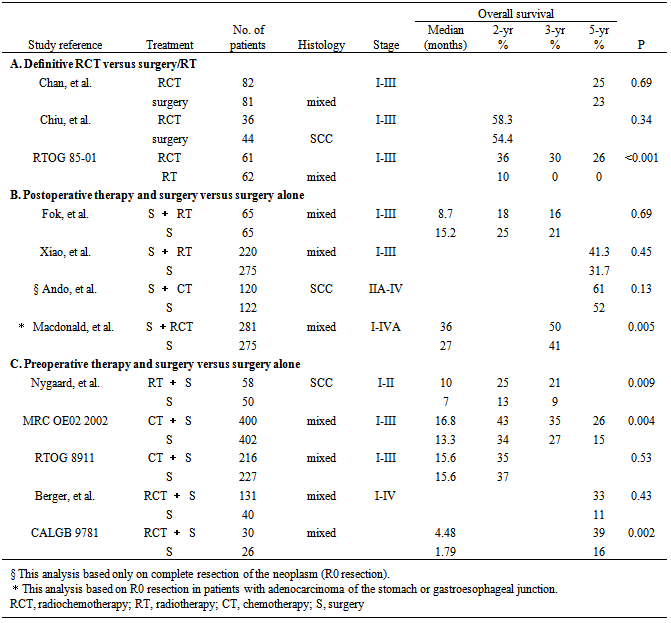
- Esophagectomy and primary radiotherapy are the basic treatment modalities of esophageal cancer. Before 1980 the 3- and 5-year survival rates after resection was poor, reported to be not exceeding 10% and locoregional tumor relapse occurred in 29% of the cases[4]. In recent decades survival figures for surgery have improved and treatment - related morbidity and mortality reduced, thanks to progress in surgical technique and intensive care. Three-field lymphadenectomy, extended radical esophagectomy with lymph node dissection of the neck, mediastinum, and abdomen, has been achieved 55.6% of overall 5-year survival in early stage esophageal cancer[5]. Contemporary approaches include transthoracic, transhiatal and minimally invasive (thoracoscopic and laparoscopic) esophagectomy depending on the tumor location, resectability rate of 70– 90% with a 5-year overall survival up to 40% and surgical mortality <5% have been shown in several trials[6-9]. Unfortunately, larger multi-institutional reports on patients undergoing resection have a median survival of only about 15 to 18 months, corresponding to a 5-year survival of 20 to 29%[10-12].Radiotherapy is applied to a broader patient population compared to surgery, however, the clinical efficacy of radiotherapy has been considered to be limited by the tolerance doses of surrounding normal tissues. Teletherapy combined with intracavity radiation – which is widely used by Japanese oncologists – has achieved significantly therapeutic effect for stage I esophageal cancer. A multicenter study showed that tumor control and overall survival rates at 1-, 2-, 5-year were 85%, 79%, 66% and 88%, 73%, 45%, respectively, in 78 cases of stage 0-I esophageal cancer by external radiation therapy alone[13]. A 5-year overall survival reached 58.9% reported by Sai et al.[14] in 34 patients of stage I esophageal cancer treated by radiotherapy alone, 2-year relapse-free-survival was 53.6% in the teletherapy group, and 79.1% when intracavity radiation was added to treatment regimen ( p = 0.05). A 52% of 5-year overall survival in stage I esophageal cancer was recently reported by Ishikawa et al.[15], tumor specific survival was elevated from 62% to 86% when intracavity radiation was combined (p = 0.04), but localregional recurrent rate reached 26.5%.A recent study from Yu et al. randomized 269 patients with operable esophageal cancer to either surgery or radiotherapy including a late-course accelerated hyperfractionated dose escalation (total dose 68.4–71.0 Gy) [16]. Five-year overall and progression-free survival was 36.9%, 23.1% and 34.7%, 20.6% for surgery and radiotherapy, respectively, and no statistically significant difference were detected. There were different reasons for treatment failure that local failure was 27.8% after surgery and 57.3% following radiotherapy; regional lymph node relapse and hematogenous metastasis were 26.6%, 20.3% and 13.3%, 13.3% in surgery and radiotherapy group, respectively. Irradiation thus has advantage of eradicating potential occult metastatic disease surrounding esophageal lesion, which is helpful for suppression of regional and distant metastases. Definitive radiotherapy for early stage esophageal cancer has fairly high efficacy, but local failure remains a major concern. Moreover, radical radiation therapy for locally advanced esophageal cancer had inferior outcome, with an overall 5-year survival rate not exceeding 10%[17].
3. Definitive Radiochemotherapy versus Definitive Radiotherapy
- Improved local control and survival were achieved in localized esophageal cancer by adding cisplatin and 5-fluorouracil (5-FU) to radiotherapy. A milestone study by the Radiation Therapy Oncology Group (RTOG) 85-01 trial compared the efficacy of radiochemotherapy with that of radiotherapy alone. 129 patients with T1-3N0-1M0 esophageal cancer were randomized to receive either combined treatment (50.4Gy with two parallel and two adjuvant cycles of cisplatin/5-FU) or radiotherapy alone. The addition of chemotherapy increased the median survival from 8.9 to 12.5 months. The 2-, 5- and 8-year overall survival was 38%, 27% and 22%, respectively, in the combined therapy group; while the 2-year overall survival was only 10% in the patients given radiotherapy alone, and none survived 5 years[18]. After a minimum follow-up of 5 years, radiochemotherapy demonstrated lower rates of locoregional failure (25% vs. 37%) and distant metastasis (30% vs. 15%).Several multi-center trails have been conducted to attempt to further improve local and distant control by dose boosting or alternate chemotherapy regime, therapeutic benefit was unfortunately not achieved. Intergroup Trial INT 0123[19] explored the outcome of dose escalation to 64.8Gy on the primary tumor, chemotherapy was identical to the RTOG 85-01 regimen, 109 patients was enrolled in both groups. 2-year overall survival and local failure were 31%, 40% and 55%, 50% in the high-dose and standard-dose group, respectively. However, the high-dose group revealed 10% of treated-related deaths, a lower frequency of deaths (2%) was noted in the standard group. A randomized phase II study (JROSG021)[20] compared the efficacy of combining short-term (cisplatin 70mg/m2 for 1 day and 5-FU 700 mg/m2 for 5 days) or protracted chemotherapy (cisplatin 7 mg/m2 for 10 days and 5-FU 250 mg/m2 for 14 days) for stages II–IVA esophageal cancer, two cycles of chemotherapy and radiotherapy (60Gy) was concurrently given. The 5-year overall and progression-free survival rates were 35%, 24% and 30%, 12% in short-term and protracted arm, respectively. Although there was no significant difference between the two arms, locoregional recurrence after 2 years was noted only in protracted arm.
4. Definitive Radiochemotherapy versus Surgery
- Evidences have demonstrated that long-term results after definitive radiochemotherapy for stage I-III esophageal cancer is comparable to that following esophagectomy[21, 22]. A retrospective study[23] showed that 82 patients were given MMC 8mg/m2, d1 + 5-FU 20 mg/kg, d1-4, 22-25 concurrently with radiation of 50-60Gy, 5-year overall and disease-free survival was 25% and 23% versus 23% and 21% in 81 patients receiving surgery only, no statistical difference was found between the two groups. A multi-center prospective study[24] randomized 80 patients with esophageal squamous cell carcinoma to either radiochemotherapy or surgery. Chemotherapy of cisplatin 60 mg/m2 (days 1 and 22) and continuous 5-FU 200 mg/m2 (days 1–42) was delivered concurrently with 50-60Gy irradiation to CTV (clinical target volume). 2-year overall survival was 58.3% and 54.4% in the radiochemotherapy and surgery group, respectively, and no significant difference was found (p = 0.34). Combined treatment regimen showed a better locoregional control and no treatment-related deaths were noted, however, perioperative mortality rate was 6.8%.In conclusion, definitive radiochemotherapy is a good alternative strategy for patients with esophageal cancer who are not suitable for surgical resection (Table 1A), and 50-60Gy is applied as the ‘‘evidence-based” dose recommendation. However, the rate of persistent or recurrent disease reaches 40-60% within 2 years following radiochemotherapy, distant control is not either satisfied. In an attempt to achieve better locoregional control and overall survival, clinical studies integrating surgery, radiotherapy and chemotherapy in variable sequences were conducted.
5. Postoperative Treatment
- In theory, postoperative irradiation can contribute to local tumor control and possibly elicit better long-term outcome. FOK et al.[25] reported that the locoregional recurrence rate was reduced from 46% to 20% (p = 0.04) at 5 years in patients who received adjuvant radiotherapy, though the difference in long-term outcome between adjuvant radiotherapy and surgery group was not significant (5-year overall survival 24% vs. 16%, p = 0.69), the median time to distant relapse for adjuvant radiation group was 9.9 months compared to the surgery-only group's 11.0 months, p = 0.76. A randomized prospective study[26] evaluated 495 postoperative patients, 220 patients who received 50-60Gy of adjuvant irradiation had a 41.3% overall survival at 5 years versus 31.7% for surgery-only group. However, the adjuvant radiotherapy arm had improved 5-year overall survival rates in node-positive (29.2% vs. 14.7%, p = 0.07) and stage III (35.1% vs. 13.1%, p = 0.003) esophageal cancer patients.
|
6. Preoperative Treatment
- Preoperative radiotherapyIt is theoretically believed that preoperative irradiation have several advantages: shrinking the tumor size to help make surgical removal easier, decreasing satellite lesions and regional lymph nodes may reduce local failure risk following curative resection, and better oxygenation in tumor area could improve the tumor response to radiation.Some studies were conducted to evaluate the effect of neoadjuvant radiotherapy in improving locoregional control and overall survival. Nygaard et al.[29] assigned 108 patients with stage T1-2NxM0 esophageal squamous cell carcinoma to neoadjuvant group and surgery only. 58 patients receiving 35Gy of irradiation before esophagectomy had a 3-year overall survival and median survival time of 21% and 10 months versus 9% and 7 months respectively, for patients underwent surgery only, a significant survival benefit was achieved, p = 0.009. A meta - analysis from RTOG-Esophageal Cancer Collaborative Group enrolled 1147 esophageal cancer patients from five randomized trials. After a median follow-up of 9 years, preoperative radiation led to an overall reduction in the risk of death of 11% and an absolute survival benefit of 3% at 2 years and 4% at 5 years, compared to surgical resection only, though the result was not conventionally statistically significant (p = 0.062)[30].Preoperative chemotherapyThe use of neoadjuvant chemotherapy for locally advanced stage esophageal cancer has been clinically accepted, and cisplatin/5-FU regimen is universally used[31]. Moreover, neoadjuvant chemotherapy provides a sensitive measurement of tumor response to certain chemical agents. The cases achieving pathological complete response (pCR) to neoadjuvant therapy are associated with significantly improved survival[32].A randomized controlled trial from Medical Research Council Esophageal Cancer Working Group[33] assessed the effects of preoperative chemotherapy on survival, dysphagia, and performance status in esophageal cancer patients. 802 patients were allocated to either two cycles of cisplatin/5-FU chemotherapy followed by surgical resection (CS group, n = 400), or resection alone (S group, n = 402). The findings showed that resection was microscopically complete in 60% of CS patients but 54% of S patients (p < 0.0001). Overall survival significantly favored the CS group, median survival was 16.8 months in the CS group compared with 13.3 months in the S group, and 2-year survival rates were 43% and 34%; p = 0.004. Postoperative complications were similar in the two patient groups.RTOG Trial 8911[34] showed that the patients receiving chemotherapy plus surgery had 59%, 35% survival rate at 1, 2 years, compared to 60%, 37% in patients underwent immediate surgery (p = 0.53). The rate of locoregional and distal failure was similar between the two groups. Neoadjuvant chemotherapy led to a higher rate of R0 resection (63% vs. 59%, p = 0.51). However, the patients with R0 resection had a 32% of disease-free survival at 5 years, only 5% of patients undergoing an R1 resection survived for longer than 5 years. Thus, improved survival can be achieved in patients with objective tumor regression after neoadjuvant chemotherapy.The benefit of neoadjuvant chemotherapy in esophageal cancer is inconclusive from evaluating several meta-analyses. Kaklamanos et al.[35] analyzed eleven randomized trials involving 2311 patients, and found that preoperative chemotherapy improved 2-year survival rate by 4.4% compared with surgery alone, though the difference was not statistically significant ( p = 0.07). The second meta-analysis published in the Cochrane Database of Systematic Reviews 2006[36] showed that preoperative chemotherapy could modestly improve survival, but failed to improve the overall rate of resections or complete resection, non-fatal complication rates were comparable to surgery alone. A recent meta-analysis[37] interpreted the impact of neoadjuvant chemotherapy in 1724 patients with local operable esophageal carcinoma, which offered a 2-year absolute survival benefit of 7% compared with surgery alone. There was no significant effect on all-cause mortality of chemotherapy for patients with squamous cell carcinoma (p = 0.12), but there was a significant benefit for those with adenocarcinoma (p = 0.014).As a whole, chemotherapy may make tumor easier to operate on and stop it spreading, and do not increase serious adverse events in patients with resectable esophageal cancer. Some evidences support that two cycles of preoperative cisplatin and fluorouracil may help the patients to live longer.Preoperative radiochemotherapySeveral randomized trails and meta-analyses compared the long-term results of adjuvant radiochemotherapy followed by surgery with immediate surgery for locoregional esophageal cancer[38-45]. The findings from Berger’s team [46] showed that preoperative radiochemotherapy not only made the tumor downstage from stage II-III to pathologic stage 0-I in 55% of esophageal cancer patients, but offered a survival benefit of 22% at 5 years (33% 5-year survival rate for adjuvant radiochemotherapy compared with 11% for surgery alone). Cancer and Leukaemia Group B (CALGB) recently released the results of study on neoadjuvant radiochemotherapy[47]. After a median follow-up of 6 years, the regimen of radiotherapy to 50.4Gy in 28 fractions plus two cycles of cisplatin/5-FU in weeks 1 and 5 before esophagectomy revealed a 5-year overall survival of 39% with a median survival time of 4.48 years, while patients received surgery alone had a survival rate of 16%, median survival time was 1.79 years (p = 0.002).Meta-analyses also suggest the beneficial effects of neoadjuvant radiochemotherapy. Urschel[48] and Fiorica[49] separately analyzed 6 identical randomized controlled trials comparing preoperative radiochemotherapy plus surgery with surgery alone for esophageal cancer. They found that a complete pathological response to radiochemotherapy occurred in 21% of patients. Compared with surgery alone, neoadjuvant radiochemotherapy significantly improved 3-year survival and reduced local-regional cancer recurrence (5% CI 0.47-0.92; p = 0.016), though 1- and 2-year overall survival rate were similar. Moreover, there was a non-significant trend toward increased treatment mortality with trimodality therapy. Australasian Gastro-Intestinal Trials Group[50] published the results of meta-analysis including 12 trails randomized comparisons of preoperative radiochemotherapy versus surgery alone (n = 1854). The hazard ratio for all-cause mortality for neoadjuvant radiochemotherapy was 0.78 (95% CI 0.70–0.88; p < 0.0001), corresponding to a 2-year absolute survival benefit of 13%; the hazard ratio for different histological tumor types was similar: 0.80 (0.68–0.93; p = 0.004) for squamous-cell carcinoma (SCC) and 0.75 (0.59-0.95; p = 0.02) for adenocarcinoma.Despite the controversy surrounding increased perioperative mortality, neoadjuvant radiochemotherapy has gained popularity as a treatment strategy for the management of locally advanced esophageal cancer, on the fact that a significant survival benefit was evident for it (Table 1C).
7. Strategies to Improve Therapeutic Efficacy
- Tumor metabolism and therapeutic responseBiological target volume—is proposed recently to stage esophageal cancer based on the quantitative assessment of tumor metabolism, higher sensitivity of determining the depth of tumor invasion and presence of regional lymph node involvement can be achieved in accurately defining radiation volume. 18F-fluorodeoxyglucose (FDG) PET/CT scan provides precise information on the location of the abnormal metabolic activity that is associated with tumors. Van Cutsem et al.[51] compared the positive lymph nodes detected by FDG PET/CT and conventional CT scanning with pathologic exam after extended esophagectomy. PET/CT had 93% sensitivity and 98% specificity, while CT had a sensitivity of 80% and a specificity of 67%, so the irradiated volume based on a positive PET/CT in a region without suspected lymph nodes on CT should also be considered. PET/CT has a big role in assessing the effect of neoadjuvant therapy, major histomorphologic response was considered as an important prognostic factor after esophagectomy. If neoadjuvant chemoradiation led to a significant reduction of FDG-uptake value in tumor sites, risk of relapse or death in patients who received subsequent surgery can be decreased by 26%[52]. Barber et al.[53] prospectively evaluated the incremental staging information, management impact and prognostic stratification of PET/CT in the primary staging of esophageal cancer. 139 consecutive patients with newly diagnosed esophageal cancer underwent conventional staging investigations, and followed by PET/CT. PET/CT changed the stage group in 56 of 139 (40%) patients and changed management in 47 of 139 (34%) patients. Of the 47 patients with management change, imaging results could be validated in 31 patients, and PET/CT correctly changed management in 26 (84%) of these. More importantly, with the accurate initial staging of patients with esophageal malignancy, oncologists can identify patients that would not benefit from an esophagectomy, and a modification of (radio-) chemotherapy can then be administered. And not only that, PET/CT with 18F-FDG has an invaluable role in assessing clinical benefit responses to treatment, optimal therapeutic concept and strength may well be evaluated to save patients from insufficient or excessive treatment.Improvemens in radiation deliveryThree-dimensional conformal radiotherapy (3-DCRT) has been extensively used in clinical practice, precise irradiation volume and reliable dose distributions in target and normal tissue can be achieved because high dose region is significantly conformed to target volume. The effectiveness of 3-DCRT has been confirmed in the treatment of nasopharyngeal carcinoma, prostate cancer and lung cancer. The anticipated benefits of 3-DCRT can also be achieved in esophageal cancer treatment, for a high dose of radiation can be given without excess damage to adjacent normal tissues such as spinal cord, heart and lung. Radiation dose to target volume can be increased by 5-10Gy while sparing more mean lung dose, and 15-25% of elevated local control rate was reported by using 3-DCRT in esophageal cancer therapy [54].Intensity-modulated radiotherapy (IMRT) has the advantages in both conformality and adherence to dose constraints. Instead of restricting useful beams to the outline of the target, IMRT takes the 3-DCRT conformal beams and divides them into many smaller subfields to control the intensity of the radiation beam to focus a higher radiation dose to the tumor while minimize radiation exposure to surrounding normal tissues. With respect to esophageal cancer, IMRT has the potential to reduce treatment toxicity even when doses are not increased, for IMRT approach can reduce the ratio of normal tissue dose to tumor dose to a minimum. Chandra and colleagues[55] compared the dose effect of IMRT with that of 3D-CRT on esophageal cancer, the items including dose distribution, dose-volume histogram and conformal index were considered. IMRT plans reduced V10 and V20 of the lungs by 10%, 5%, respectively; and the mean lung dose was decreased by 2.5Gy.Image guided radiotherapy (IGRT) is an advanced method for guiding the IMRT to the target area. On the one hand, a tumor may have movements between treatments in response to the position of other organs or the patient's breathing, using imaging equipment, the doctor is able to obtain daily high-resolution imagery to precisely pinpoint the tumor location immediately prior to treatment. By imaging the tumor daily, on the other hand, the tumor size or location can be detected in the process of radiation treatment which permits the doctor to locate the tumor or modify treatment plan. This allows delivery of higher radiation doses to targeted tumors and a minimized dose to healthy tissues outside of the target volume. Study[56] showed that when IGRT is used for esophageal cancer treatment, daily IGRT can eliminate positioning errors, underdosing of the CTV and excess dose to normal structures, thus conformal radiotherapy and precise dose can be optimally delivered to the tumor sites.Molecular targeted therapyFor esophageal cancer, potential tumor targets/markers include those related to growth regulation (epidermal growth factor receptors [EGFR] and Ki-67), angiogenesis (vascular endothelial growth factor [VEGF]), inflammation (cyclooxygenase [COX]-2 pathway), cell cycle control (p16, p21, and cyclin D1) and apoptosis (p53, Bax, and Bcl-2). Some of study results have been translated into clinical practice, the most prominent example is targeting of EGFR in combination with radiotherapy/chemotherapy. Cetuximab is an IgG1 monoclonal antibody that inhibits ligand binding to the EGFR 5-7 and stimulates antibody-dependent cell-mediated cytotoxicity. Good clinical response achieved by definitive chemoradiation plus cetuximab in esophageal cancer have been reported in several phase II trials. Safran et al[57]. assessed cetuximab, paclitaxel and carboplatin weekly for 6 weeks with 50.4Gy radiation in 60 esophagogastric cancer patients, and found that this regimen had a 70% clinical complete response. The GERCOR phase II trial[58] enrolled 79 patients with locally advanced esophageal or cardia cancer, all patients received 2 cycles of FOLFOX induction therapy plus cetuximab, then radiotherapy 50.4 Gy with FOLFOX plus cetuximab. After a median follow-up of 19.4 months, overall response rate was achieved in 77% of patients, and the median progression-free survival (PFS) was 13.8 months. In a single-arm trial of cetuximab, paclitaxel, cisplatin and concurrent radiation in 31 patients with stage II–IVa esophageal squamous cell carcinoma, a 69.0% clinical complete response was observed, the 1- and 2-year PFS rates were 85.5% and 75.1% over a median follow up of 23.6 months[59]. Moreover, cetuximab was well tolerated in these studies. The above encouraging results suggest potential activity and minimal toxicities with EGFR antibodies for esophageal cancer.
8. Conclusions
- The data to support the use of radiation alone for curative intent are limited, and definitive chemoradiation is a good strategy for the treatment of locally advanced unresectable esophageal cancer, but local control remains a problem, and it is warranted to explore potential ways of improving local control. Preoperative radiochemotherapy trends toward increased survival over preoperative chemotherapy alone, and neoadjuvant radiochemotherapy provides a significant benefit over surgery alone for esophageal cancer. Molecular imaging has dramatically improved our ability to stratify patients who will benefit from surgery, radiation, chemotherapy, or multimodality treatment. Better tumor volume delineation can also be led by metabolic imaging, and the advances in improved computed tomography-based software allow more precise planning and delivery radiation. With the benefits of these advances, individualized therapeutic approach can be successfully transferred to enhance tumor responses and limit treatment-related toxicity of this common disease.
ACKNOWLEDGEMENTS
- This work was supported by the National Natural Science Foundation of China (No: U1204816).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML