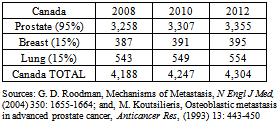-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Tumor Therapy
p-ISSN: 2163-2189 e-ISSN: 2163-2197
2013; 2(1): 10-17
doi:10.5923/j.ijtt.20130201.02
Economic and Clinical Net Benefits from the Use of Samarium Sm-153 Lexidronam Injection in the Treatment of Bone Metastases
D. Wayne Taylor
The Cameron Institute and McMaster University, Hamilton, L7L 5R8, Canada
Correspondence to: D. Wayne Taylor, The Cameron Institute and McMaster University, Hamilton, L7L 5R8, Canada.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
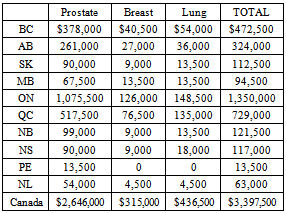
Samarium Sm-153 Lexidronam Injection (Samarium-153) is used to treat bone pain that results when cancer spreads to the bone. It has been on the market since 1997 but still is not funded by any one of the 10 provincial cancer care authorities in Canada. The only cost-effectiveness study using randomized control trial data of Samarium-153 for pain relief in patients with bone metastases clearly demonstrated that Samarium-153 was a dominant therapy. At the time of writing, the product cost of Samarium-153 for one patient treatment was $4,500. At that price, in 2012, the total product cost for all of the projected 755 patient treatments in Canada would be $3,397,500. For an investment of $3,397,500 in Samarium-153, system savings of $7,758,905 could be realized for a return on investment (ROI) of 228%. Overall treatment cost-savings would have accrued while Samarium-153 provided suffering cancer patients with equal or better care and clinical outcomes, as measured by pain relief and quality of life.
Keywords: Samarium Sm-153, Bone Pain, Palliative Care, Cost-Effectiveness
Cite this paper: D. Wayne Taylor, Economic and Clinical Net Benefits from the Use of Samarium Sm-153 Lexidronam Injection in the Treatment of Bone Metastases, International Journal of Tumor Therapy, Vol. 2 No. 1, 2013, pp. 10-17. doi: 10.5923/j.ijtt.20130201.02.
Article Outline
1. Introduction
- Healthcare is about money. Despite all the protests by politicians, bureaucrats, payors and providers that healthcare is about providing the right treatment to the right patient at the right time, to do that requires money - and lots of it. Yet most healthcare systems in the world do not seem to have enough funding today thus putting into jeopardy the mantra of the politicians, bureaucrats, payors and providers. Canada is a case in point where palliative care for cancer patients is, in one case, 15 years behind current practice. Money trumps best practice of care. Yet it is not the want for money that is the problem; it is the inertia of the system to reallocate existing funds.Around the world there are basically four types of healthcare system[1]:
1.1. European Model
- Comprehensive universal healthcare funded largely with tax monies and/or government mandated insurance (80-90% of total expenditure) supplemented with patient co-payments and complemented by a smaller parallel private-pay healthcare system (10-20%);
1.2. Developing Countries Model
- Private healthcare for the small but rich, ruling classes (80-90% of expenditure) and limited public healthcare for the poor majority (10-20%);
1.3. American Model
- Most insurance is private yet the split of services financing is about even between private and public (medicare - federal government mandated coverage for seniors; medicaid - state-administered care for low income individuals and families; s-chip[state children’s health insurance program], again, is state-administered coverage for uninsured working poor and lower, middle class children; va[veterans’ administration] is the healthcare system for veterans of the us armed forces; and,
1.4. Canadian Model
- All medical doctors and hospitals are paid out of tax monies (70%) and the rest of the system is private (30%)[2].Canada is unique within the healthcare universe in its distinctive split between public and private financing. But regardless of design, almost all healthcare systems wrestle with balancing the three points of the Iron Triangle (see Figure 1)[3].“Canadian healthcare” is really a patchwork quilt of 17 different provincial (10 - British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, New Brunswick, Nova Scotia, Prince Edward Island, Newfoundland and Labrador), territorial (3 - Nunavut, Northwest Territories, Yukon ) and federal (4 - Department of National Defence, Veterans’ Administration, the federal government, and the Non-insured Health Benefits for First Nations and Inuit) healthcare systems each with its own take on balancing the Iron Triangle.
 | Figure 1. The Iron Triangle |
2. Samarium Sm-153 Lexidronam Injection (Samarium-153)
- Samarium Sm-153 Lexidronam Injection (Samarium-153) is a member of the class of pharmaceuticals known as “radiopharmaceuticals” – drugs that contain a radioactive ingredient. Radiopharmaceuticals are a type of “systemic” therapy in which one dose of a drug simultaneously targets multiple sites of metastasis, and they have been used since 1950s to provide pain relief for cancer that metastasizes to bone. Samarium-153 is used to treat bone pain that results when cancer spreads to the bone. Generally, when cancer has metastasized to the bone it is characterized by either the breakdown of the bone (osteolytic), the formation of bone (osteoblastic), or both. Myeloma (or multiple myeloma) is the name given to a cancer that springs from the plasma cells in the bone marrow when these plasma cells form abnormal antibodies. These antibodies, in turn, can damage the bone, the bone marrow and other organs. Myeloma usually results in osteolytic lesions and excessive bone destruction.A much more common cancer, prostate cancer, is usually accompanied by osteoblastic lesions and excessive bone growth[7]. Two trials have shown that 84% - 92% of patients with metastatic, castrate-resistant, prostate cancer have radiographic evidence of bone metastases[8],[9].Most bone metastases exhibit both osteolytic and osteoblastic characteristics – two extremes of a broad spectrum. Studies in the US have confirmed that patients with osteoblastic lesions may benefit from systemic treatment with Samarium-153. Samarium-153 was approved in 1997 by the Food and Drug Administration (FDA) in the United States (US) for pain relief in cancer patients with confirmed osteoblastic, metastatic bone lesions that are enhanced on a radionuclide bone scan. Approximately one-half of patients with bone metastases are symptomatic with debilitating pain[10],[11].In 2004, the Programme in Evidence-Based Care of Cancer Care Ontario reported that Samarium-153 “may be considered as an option for the palliation of multiple sites of bone pain from metastatic prostate cancer” and “may also be considered for patients with lung and breast cancers”[12].Alberta Health Services released their Palliative Radiotherapy Clinical Practice Guideline PAL-001 in 2008 (revised in 2010) stating that Samarium-153 was “effective in providing pain relief” from bone metastases[13].
3. Clinical and Quality-of-Life Implications of Osteoblastic Metastatic Lesions
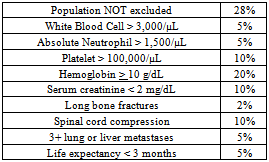
- Cancer that occurs in a human organ and subsequently spreads to bone is called metastatic bone disease (MBD). With much improved treatment of many organ cancers - such as prostate, breast and lung - patients are surviving and living longer except when patients develop bone metastases. In fact about half of organ tumours can metastasize to the skeleton[14]. Once tumours metastasize to bone they are usually incurable[15].Metastases are the most common amongst malignant bone tumours with most involving the axial skeleton (skull, spine and pelvis) and 90% being multiple. MBD spreads hematogenously, occurring most frequently where red bone marrow exists. The primary carcinomas that most often metastasize to bone (80% of the total) are:● Prostate (60% of metastases in men);● Breast (70% of metastases in women);● Lung; and● Kidney/renal cell.Others include thyroid, stomach and intestines.Most lesions are asymptomatic but, when they are symptomatic, pain in the spine, pelvis or extremities is the major concern.Metastases that are usually purely osteoblastic are:● Prostate;● Medulloblastoma; and● Bronchial carcinoid. Lung and breast are usually both, osteoblastic and osteolytic.Besides the regular morbidity, mortality and quality-of-life issues surrounding cancer, MBD can also lead to pathologic fractures and/or spinal cord compression.Bone scans are the preferred diagnostic tool as 10% - 40% of lesions will not appear on traditional radiographic film [16]. Treatment options may include surgery, chemotherapy, external-beam radiation therapy, endocrine therapy, radioisotope therapy with radiopharmaceuticals such as Samarium-153, or a combination of these.Patient candidates for radioisotope therapy should have platelets >100,000L and a white blood cell (WBC) count >2,500L to counter any bone marrow suppression. Good kidney function is also preferred as urination is the primary route of excretion for radiopharmaceuticals[17].
4. Business Impact Studies
- Healthcare economic studies usually look at three types of costs: direct costs, both medical and non-medical; indirect costs, such as lost wages due to illness; and intangible costs which may include psychosocial and societal costs.A “business impact study,” such as this one, is only concerned with direct costs. Direct costs are generally much greater for inpatient treatments than outpatient treatments. In fact, hospitalization costs are the single largest contributor to incremental health costs today in both Canada and the US.Almost all of the available literature reviewed was American. However, these studies are still very instructive for Canadians because they focused on Medicare patients. Today the split of health services in the US is about even between public (Medicare, Medicaid, S-CHIP and the VA) and private. US Medicare is, broadly speaking, federal government mandated insurance coverage for seniors. The difference between Medicare reimbursement fees and private insurance “billed charges” in the US are significant given that private insurance billed charges in the US generally must also include allowances for bad debt, uncompensated care, and capital costs which Medicare does not reimburse. US Medicare reimbursements simply cover the direct cost of care and are more in line with Canadian reimbursement figures, rather than private insurance billed charges, thus making the economics of these studies very comparable to the Canadian cost reimbursement situation.In the US, insurance coverage of Samarium-153 varies. Samarium-153 is covered by Medicare Part B and Medicaid in the US (these latter two being important comparators when analyzing the business case for Samarium-153 in Canada). For reimbursement, insurers usually require a recent bone scan (within a month or so) and the patient must be experiencing pain; pain relief is generally the end point.
5. Treatment of Osteoblastic Bone Lesions – Clinical and Economic Outcomes
- Over-the-counter analgesics, such as aspirin and acetaminophen, and prescription-only drugs such as morphine, oxycodone and other opioids are common drugs that can provide general pain relief. Opioids have the potential unpleasant side effects of constipation, nausea, vomiting, drowsiness, cognitive impairment and addiction [18].Samarium-153 is often administered in conjunction with traditional analgesics and opioids. Over time patients taking Samarium-153 were able to reduce their consumption of opioid analgesics[19],[20],[21] by as much as 60% - 69% and 22% - 32% discontinued opioid analgesics altogether[22]. Patients on placebo increased their intake of opioids as much as 26%[23]. Patients initially treated for bone pain with placebo and then switched to Samarium-153 had improved outcomes with 57% - 65% of them receiving some degree of pain relief that they had not before[24].The first radiopharmaceuticals were Phosphorous-32 and Strontium-89. Samarium-153 combines a radionuclide (an atom characterized by the composition of its nucleus, i.e. the quantity of protons, neutrons and energy) with more desirable nuclear properties (shorter half-life, lower energy-particle emissions) and greater selectivity than these earlier radiopharmaceuticals. Strontium-89 has a half-life of 50.6 days[25] whereas Samarium-153 has a half-life of 1.93 days, or 46 hours[26]. This is an important characteristic of Samarium-153 for patients as an isotope’s half-life, in part, determines its toxicity profile. A longer half-life translates into less-reversible myelosuppression[27].Biphosphonates, that prevent the loss of bone mass, target bone destruction while Samarium-153 targets bone formation. Samarium-153 accumulates more in osteoblastic lesions than it does in normal bone, with a lesion-to-bone ratio of approximately 5:1. Although Samarium-153 has been on the market for 15 years in the US it is considered to be one of the “next generation” of radiopharmaceuticals which offer clear advantages to the patient in treating pain associated with bone metastasis, as well as to the provider and the funder:● Single dose injection lasting only a few minutes, usually on an outpatient basis;● Onset of pain relief, amongst patients who respond, within one week of injection and with maximum pain relief generally achieved within 3 - 4 weeks;● Significant reduction in bone pain scores in patients –○ suffering from prostate, breast and other cancers;○ newly diagnosed with myeloma or bone metastasis;○ not responding to other treatments; and,○ in whom cancer returned after initial successful treatment[24],[27].In one clinical trial, 74% of patients receiving the approved dose of Samarium-153 experienced pain relief within 4 weeks and lasting for 12 weeks[28]. Two-thirds of those patients who responded positively to Samarium-153 were still experiencing pain relief at 16 weeks. Another inpatient study reported adequate pain control for 78% - 95% of patients[29]; yet another reported 83% - 86% positive response[21].Samarium-153 is not without side effects. Bone marrow toxicity – a decrease in the body’s number of blood cells – occurred in 33% of patients in one randomized phase III clinical trial[20]. As a result it is not typically recommended that Samarium-153 must be administered to patients at the same time as chemotherapy or external-beam radiation therapy. A reduced white cell count is often accompanied by the increased risk of infections. A reduced platelet count lessens blood clotting. Non blood-related side effects and their occurrence rates were: pain flare (7%); infection (7%); spinal cord compression (6.5%); diarrhea (6%); arrhythmias (5%); and blood in the urine (5%)[30].As stated above, Samarium-153 is usually not administered at the same time as chemotherapy or external-beam radiation therapy. There is some evidence, though, that Samarium-153 can be administered simultaneously with chemotherapy safely and efficaciously [31],[32],[33]. For about 12 hours after injection, radioactivity will be present in the patient’s urine requiring appropriate care to be taken[33]. Samarium-153 can also be harmful to fetuses and infants therefore it should not be administered to pregnant or nursing mothers. Samarium-153 is an ideal pain reliever, where indicated, for patients in hospice care[33].The Health Technology Inquiry Service (HTIS) of the Canadian Agency for Drugs and Technologies in Health (CADTH) recently conducted a literature search on the clinical and cost-effectiveness of radiopharmaceutical therapy for the treatment of metastatic bone pain[34] and identified six articles published between 2002 and January 2009 that are reviewed below in descending order of strength of evidence.A 2005 systematic review of the English published literature by Bauman et.al., of the London Regional Cancer Centre in Ontario, concluded that significant differences in response favoured the use of Samarium-153 as an option for the palliation of multiple sites of bone pain from metastatic cancer where pain control with conventional analgesic regimens was unsatisfactory and where activity was demonstrated on a bone scan of the painful lesions[35].Another 2005 systematic review, this time conducted for the National Health Service (NHS) in the United Kingdom (UK) and published in Lancet, reported palliation response rates between 40% and 95% for Samarium-153, with pain relief commencing within 1 - 4 weeks and lasting for up to 18 months[36].The only cost-effectiveness study using randomized control trial data of Samarium-153 for pain relief in patients with bone metastases clearly demonstrated thatSamarium-153 was a dominant therapy[37].The cost for pain control using conventional therapy was €12,500 in 2005; the cost for Samarium-153 was €5,600. Assuming the average 2005 CND/€ currency exchange rate to have been CND 1.4825811142 / € 1.0[38], and healthcare inflation for 2005-2011 to have been 5% per annum[39], in 2012 Canadian dollars the equivalents would be $26,075 for conventional palliative care and $11,680 usingSamarium-153 for a savings of $14,395 - a greater than 2:1 multiple cost-effectiveness advantage. Savings would be in inpatient hospital overhead costs, staff time, differential-in-price of more expensive less effective products, materials, management of side effects, and other ancillary treatment costs.These numbers are in line with previous findings[40] for an earlier radiopharmaceutical, strontium-89, but with Samarium-153 providing better clinical outcomes as measured by pain relief and quality of life (QOL), and much better safety and side effect profiles given the much shorter half life of Samarium-153.A 2004 article by Sartor et.al. documented the results of a phase III randomized trial designed to assess the effectiveness of Samarium-153 for palliation of bone pain in patients with hormone-refractory prostate cancer[26]. A sample totaling 152 men with hormone-refractory prostate cancer and painful bone metastases were enrolled in a prospective, randomized, double-blind trial comparing radioactive (153Sm) versus nonradioactive (152Sm) lexidronam complexes with patients randomized 2:1 to the radioactive (153Sm) agent. The evaluation period was 16 weeks. Samarium-153 exhibited positive effects in pain relief, compared to the placebo, within 1 to 2 weeks. Reductions in opioid use were recorded at weeks 3 and 4. Mild, transient bone marrow suppression was the only adverse event. The mean nadir white blood cell and platelet count (3 to 4 weeks after treatment) was 3,800/µL and 127,000/µL, respectively. Counts recovered to baseline after approximately 8 weeks. No grade 4 decreases in either platelets or white blood cells were documented. Overall, Samarium-153 was determined to be both safe and effective in the palliation of pain associated with bone metastases.A smaller, earlier study by Wang et.al. determined the therapeutic efficacy of Samarium-153 was 77.8% (almost twice as efficacious as pamidronate disodium at 44.4%) with mild, transient, reversible myelosuppression wherein white blood cells and platelets had recovered within 6 weeks[41]. Since the HTIS/CADTH literature review several studies have been conducted. One, in 2007, studied 46 patients with breast cancer and painful bone metastases. In this study, 80.4% of the patients experienced statistically significant pain reduction with the use of Samarium-153 at 4, 8, 12 and 16 weeks post-therapy (p<0.0001).A multi-centre, phase IV study reported in 2007 that treatment with Samarium-153 for metastatic bone pain from prostate, breast, lung and other primary carcinomas could be repeated without significantly increasing toxicity[42].Finally, a report published in 2009 concluded Samarium-153 was as good as, if not better than, opioids in the treatment of pain secondary to bone metastases, and improved the quality of life in the last year of life for patients, without the side effects associated with opioids[19]. The study assessed whether baseline and short-term QOL (the proxies for which were pain and treatment side effects at 0, 4 and 8 weeks) differed in patients with symptomatic metastatic prostate cancer undergoing palliation by opioids, nonsteroidal anti-inflammatory agents (NSAIDs), strontium-89 chloride, and Samarium-153. Patients were grouped by which of the above palliative options was their primary palliative intervention. The study found that pain increased in those patients who were administered NSAIDs (21%) and strontium-89 (46%) while pain decreased by 27% in patients treated by Samarium-153 and by 27% in patients treated by opioids.This study by Papatheofanis, Smith and Najib was also significant for another reason. Even though opioids are the standard palliative therapy for pain secondary to bone metastases[43] there are numerous and frequent side effects from prolonged opioid use, as discussed above. The fear of these well-known side-effects also led to patient noncompliance with an opioid regimen. Therefore, in terms of the effect that various palliative interventions had on overall QOL for these cancer patients, Samarium-153 was clinically the preferred choice[44].Although survival has not been studied specifically, the researchers in one study did observe that survival improved by 50% with higher doses of Samarium-153 (9 months) than with lower doses (6 months)[29].
6. Business Impact of Samarium-153 for Canadians
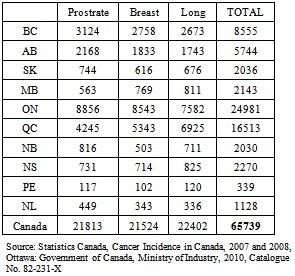
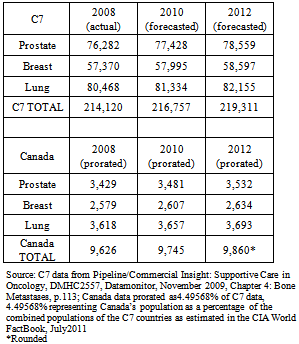
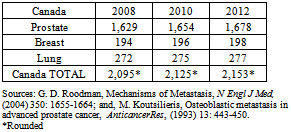
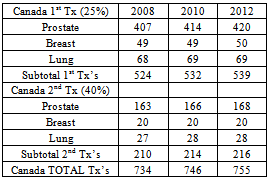
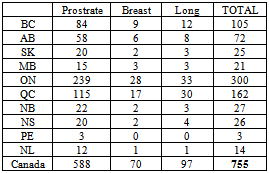
- In 2007, there were 65,739 reported new cases of prostate, lung and breast cancer in Canada (see Table 1). Regrettably, the incidence of bone metastases associated with these cancers was either not captured or not publicly reported. Therefore these figures had to be calculated by prorating the actual 2008 incidence and the forecasted incidence for 2010 and 2012 as reported for seven other countries: the US, Japan, Germany, France, the UK, Italy and Spain thus yielding 9,860 (~15%) bone metastases in 2012 (see Table 2). According to Roodman[45], 95% of bone metastases in prostate cancer are osteoblastic; 15% are osteoblastic in breast cancer; and 15% for lung cancer. Applying these percentages to the numbers in Table 2 yields the number of osteoblastic bone metastases for each of the three cancer sites in question for a total of 4,304 in 2012 (see Table 3).
|
|
|
|
|
|
|
|
7. Conclusions
- Overall treatment cost-savings would have accrued while Samarium-153 provided suffering cancer patients with equal or better care and clinical outcomes, as measured by pain relief and quality of life.
ACKNOWLEDGEMENTS
- The British Columbia Institute of Technology (BCIT) is acknowledged for its financial support of this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML