-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Tumor Therapy
p-ISSN: 2163-2189 e-ISSN: 2163-2197
2012; 1(4): 20-25
doi: 10.5923/j.ijtt.20120104.01
High-Intensity Focused Ultrasound (HIFU) For the Prostate Cancer: 5-year Experience
Vyacheslav Solovov, Leonid Shaplygin, Mikhail Vozdvizhenskiy, Ravil Khametov
Department of Interventional Radiology, Samara Regional Oncology Center, Samara, 443031, Russia
Correspondence to: Vyacheslav Solovov, Department of Interventional Radiology, Samara Regional Oncology Center, Samara, 443031, Russia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
HIFU shows a successful treatment for localized prostate cancer. Here we explored the effectiveness of the HIFU treatment for the prostate cancer, hormone-resistant prostate cancer and failure after external beam radiotherapy and radical prostatectomy. 795 patients were treated in our centre in 2007 – 2012: Kaplan-Meir analyses of the total group indicated that the risk of progression was 23% after 5 years of follow-up. Our experience shows that HIFU ablation is safe, minimally invasive, effective treatment with moderate side effects for the PC, hormone-resistant prostate cancer, HIFU also may be used as a salvage therapy.
Keywords: HIFU, High Intensity Focused Ultrasound, Prostate Cancer
Cite this paper: Vyacheslav Solovov, Leonid Shaplygin, Mikhail Vozdvizhenskiy, Ravil Khametov, "High-Intensity Focused Ultrasound (HIFU) For the Prostate Cancer: 5-year Experience", International Journal of Tumor Therapy, Vol. 1 No. 4, 2012, pp. 20-25. doi: 10.5923/j.ijtt.20120104.01.
Article Outline
1. Introduction
- Prostate cancer (PC) in developed countries is the most common malignancy among men and the second leading cause of cancer death after lung cancer[1]. In 2010, PC took the third place in the structure of cancer among male population of Russia (11.0%) - showed 26,268 new cases of the disease[2]. Over the last 10 years the increase was 155,3%. 10,251 patients died from prostate cancer in 2010 (fourth place on the men’s deaths from cancer). Radical prostatectomy (RPE) and external beam radiation therapy (EBRT) are the standard treatments for patients with localized prostate cancer with a life expectancy of at least 10 years[3]. Patients presenting with localized prostate cancer are treated with curative intent by surgery or radiation, but up to 30% will relapse. Treatment then involves androgen- ablation therapies and all patients will eventually develop hormone-resistant prostate cancer (HRPC). In the past, systemic treatments for HRPC, such as second line hormone therapy, chemotherapy, mitoxantrone and prednisone, offered palliative benefit, but no survival advantage[4]. Newer treatments with docetaxel and prednisone have shown to offer both palliative and survival benefits[5, 6].During the last decade new minimally invasive therapeutic modalities for prostate cancer have developed, such as brachytherapy, HIFU and cryotherapy. HIFU is an alternative choice in localized and low or intermediate- risk prostate cancer treatment[7]. Rising PSA in nonmetastatic prostate cancer indicates failure of initial local therapy and the onset of early HRPC cancer prior to documented clinical metastases. The ideal salvage therapy for these patients is not clear and includes salvage local therapies and systemic approaches, of which the mainstay is hormonal therapy. Treatment needs to be individualized, based upon the patient's risk of progression, the likelihood of success and the risks involved with the therapy. Therefore attention of scientists is focused on the developed and implemented into clinical practice new effective, minimally invasive treatments for prostate cancer, HRPC and failure after EBRT and RPE[6]. However, due to the fact that studies analysing the effectiveness of HIFU-therapy for prostate cancer HRPC and failure after EBRT and RPE with a cohort of sufficient size and statistical power is few, the real work done. The main aim of this study is to evaluate the results of HIFU treatment of PC with low and high risk progression, and local recurrence after EBRT and RPE.
2. Main principles of HIFU
- The first therapeutic trial of high intensity ultrasound beams was carried out in 1942[8]. The Fry brothers were credited with the first application of HIFU for neurologic disorders in humans[9]. High-energy ultrasound, parabolic focused on tissue leads to mechanical alteration of the cells and causes changes in biological structures (Figure 1). During application of focused ultrasound three different physical mechanisms can be observed: mechanical, thermal and cavitation effects[10].Mechanical effects are induced by sudden pressure increase within the tissue by the HIFU beam being highly energetic.
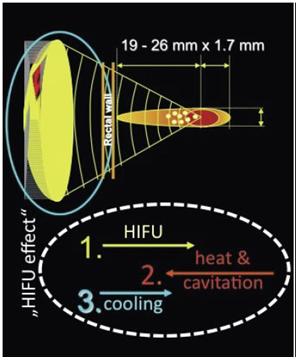 | Figure 1. Physical principle of focused energy application |
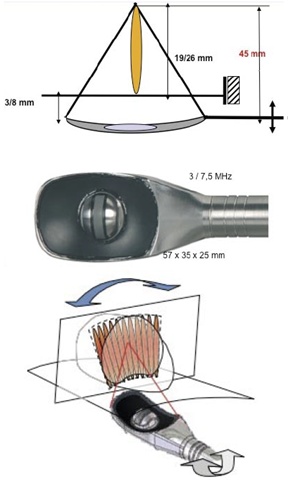 | Figure 2. Focal point adjustment: A) Penetration depth (19-26 mm; B) Latero-longitudinally (1.7 mm steps) |
 | Figure 3. HIFU devices: Ablatherm® |
3. Materials and Methods
- Seven hundred ninety five patients with PC underwent HIFU in the period between September 2007 and August 2012. Every patient was available for oncological follow-up. Inclusion criteria were: patients with prostate localized and locally advanced PC, patients after EBRT or RPE failure. Exclusion criteria were: anal stenosis, metastatic PC. The oncology follow-up consisted of PSA evaluation, MRI and transrectal biopsy in the case of rising PSA. 139 patients were hormone-resistance (median time before hormone- resistance 25 months), 297 – received neoadjuvant hormone therapy 6 months, 320 – no treatment before HIFU, 39 – after the EBRT and RPE failure. 706 patients underwent trans-urethral resection of prostate (TURP) and HIFU-procedure; 89 underwent only HIFU ablation (prostate volume <40cc). All patients underwent spinal anaesthesia. We used the Ablatherm® device (EDAP, Lyon, France).
4. Results
- In this paper, we analyse our 5 year experience with HIFU treatment of 795 patients with PC. The patients were divided into three groups according to the cancer progression risk: low risk group - 465 patients, Gleason ≤7, stage T1-2N0M0, age 69 (60-89) years PSA before treatment 40,0 (5,8-92,9) ng/ml, mean prostate volume - 39,3 (28-92) cc; high risk progression group – 291 patients, Gleason ≤9, stage T2-3N0M0, age 72 (52-83) years, PSA before treatment 30,3 (20,1-60) ng/ml, mean prostate volume - 41,2 ( 25-198) cc, the third group - patients after EBRT and RPE failure – 39 patients. Cancer clinical staging in the whole group was T1 in 149 patients, T2 in 321 patients and T3 in 325 patients. Histological Gleason score was 2 in 42 patients, 3 in 87 patients, 4 in 113 patients, 5 in 136 patient, 6 in 189 patients, 7 in 158 patients, and 8 in 62 patients, 9 in 8 patients. The average volume of treated prostate tissue was 30 cc (range 5-38.4). High-intensity focused ultrasound treatment had a mean duration of 120 minutes (range 60-245).The average hospital stay was 7 days. 251 patients underwent TURP+HIFU in the same session. 455 patients with prostate volume larger than 60 cc. first underwent TURP and then HIFU after one month. 89 patients with small prostate volume underwent only HIFU. At the end of the procedure a Foley catheter was placed.
|
|
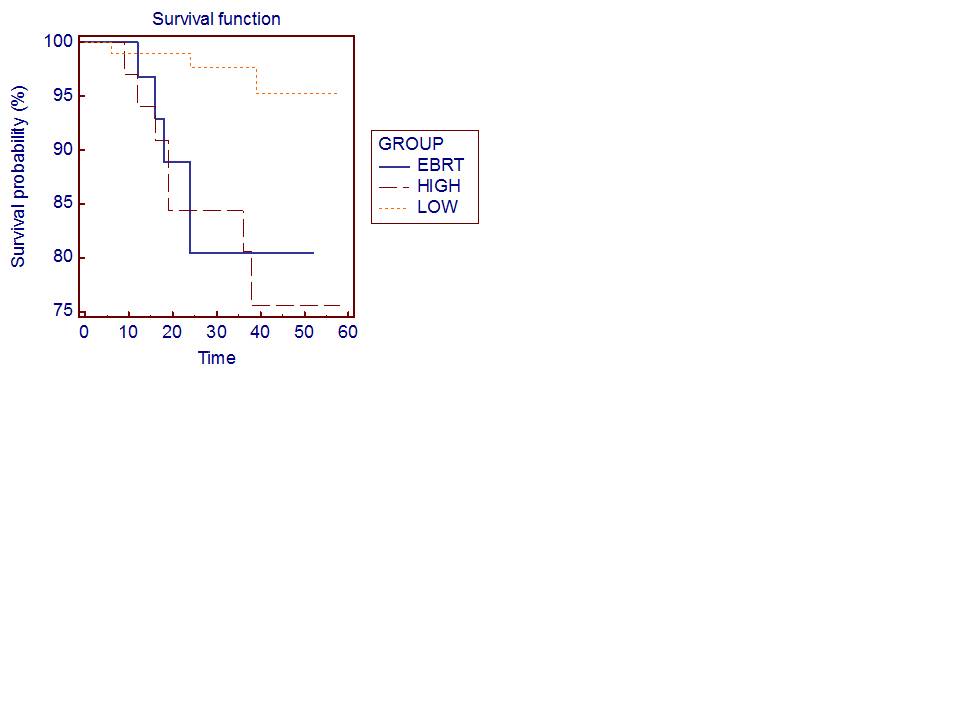 | Figure 4. Kaplan–Meier disease-free survival (DFS) curves according to risk group after HIFU |
5. Discussion
- Treatment for PC may include: active surveillance, interstitial prostate brachytherapy, EBRT and RPE. There is still ongoing debate on the efficiency of focal treatment, but at the same time different focal options emerge. Brachytherapy and radiation external beam therapy are the most used as minimally invasive techniques, not only for the therapy of localized PC but also for the palliation of high-grade tumours. Some medical associations recommend HIFU for treatment of PC, but its accuracy is still not clear. Prostate cancer is dependent on the presence of androgens. Patients that are not suitable for radical surgery and with metastatic disease are typically first treated with hormonal ablation: strategies include testicular androgen deprivation by either bilateral orchidectomy or administration of a luteinizing hormone releasing hormone (LHRH) agonist, and treatment with anti-androgens such as flutamide to compete with testosterone for the androgen receptor binding site. Unfortunately, resistance to androgen suppression invariably develops: cells accumulate further genetic abnormalities and proliferate despite low testosterone levels at a median interval of 12-16 months after initiation of endocrine treatment. Subsequent lines of hormonal therapy act through related pathways and include the use of the synthetic oestrogen, the reduction of adrenal androgen production by administration of glucocorticoids. There are limited treatment options once recurrent prostate cancer develops androgen independence. Palliative chemotherapy with docetaxel has been shown to improve survival and is commonly instituted for metastatic disease following failure of maximal androgen blockade but is not suitable for all patients, particularly those with poor performance status. Those patients with no metastatic disease may receive local salvage treatment such as brachytherapy or HIFU.In Russia, localized prostate cancer, when possible to conduct RPE, detected only 35% of patients[1]. In this case, among patients with stage I-II after radical prostatectomy or radiation therapy in 25-50% of cases prostate cancer recurrent is developed[32]. Therefore, patients are not suitable for surgery or radiation therapy, and with recurrent prostate cancer assigned to hormone therapy: bilateral orchiectomy or maximum androgen blockade. It is noted that the rate of relapse-free and overall survival of patients with prostate cancer have remained unchanged for several decades (12-24 and 24-36 months respectively)[33]. The second and the third lines of hormone therapy, chemotherapy are effective only in 15-20% of cases and do not lead to a significant increase life expectancy of patients, while possessing significant side effects[4, 27, 28, 30].It should be noted that at present tactics and strategies of prostate cancer treatment has not been developed in accordance with the implemented in the practice minimally invasive new technologies, there are no clinically based recommendations. Publications of focal prostate cancer therapy are few, they are based on small clinical material, have a short period of observation, and do not define the role and the place of HIFU-therapy in the treatment of prostate cancer. To date, long-term[31] and the medium-term results published[32, 33] about HIFU-therapy of prostate cancer. According to a European multicenter study that included 559 patients with prostate cancer in low-and moderate-risk, Thüroff et al.[30] reported a negative biopsy result after the HIFU-therapy in 87.2%. Blana et al. evaluated the results of HIFU in 146 patients with a mean follow-up of 22.5 months median preoperative PSA was 7.6 ng / mL, while the median PSA level at 3 months after therapy was 0.07 ng / mL[29].We analysed the results of HIFU treatment of 795 patients with prostate cancer. The estimated 5-year disease-free survival Kaplan-Meer had 95.5% efficiency of HIFU therapy in the group with low risk of progression and 75% in the group with high risk of progression. Treatment results showed that, in general HIFU-therapy was successful in 90.9% of patients. At the same time there were moderate short-term side effects. However, it was obvious that a more long-term monitoring of the effectiveness of HIFU therapy in patients with prostate cancer were necessary.
6. Conclusions
- The most recent publications concluded that the use of HIFU is an effective standard treatment for prostate cancer with a broad range of indications in all tumour stages: in the primary treatment of local prostate cancer, in patients with local recurrence after failure of any primary treatment, and as an adjuvant therapy in the palliation of systemic prostate cancer.Our experience shows that HIFU is safe, minimally invasive, effective in treatment for localized and locally advanced prostate cancer, after EBRT and prostatectomy.
References
| [1] | Jemal A. et al. “Gender Statistics”. CA Cancer J. Clin, July 7, 2010. |
| [2] | Chissov V, Starinsky V, Petrova G. “Malignant neoplasms in Russia in 2010 (morbidity and mortality)”, M. FGBU "MNIOI by Herzen "Ministry of Russia”, Russia, 2012. |
| [3] | Prostate Cancer Treatment Guidelines, NCCN, v.1.2012. |
| [4] | Tannock IF, Osoba D, Stockler MR, et al. “Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points”. J Clin Oncol, 1996, Vol.14, p.1756–64. |
| [5] | Petrylak DP, Tangen CM, Hussain MH, et al. “Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer”, N Engl J Med, 2004, Vol.351, p.1513–20. |
| [6] | Tannock IF, de Wit R, Berry WR, et al. on behalf of the tax 327 Investigators. “Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer”, N Engl J Med, 2004, Vol.351, p.1502–12. |
| [7] | Thuroff S, Chaussy C, Vallancien G, et al. “High-intensity focused ultrasound and localized prostate cancer: efficacy results from the European multricentric study”, J Endourol, – t.17 (8), p.673-7, 2003. |
| [8] | Lynn JG, Zwemer RL, Chick AJ, Miller AE. A new method for the generation and use of focused ultrasound in experimental biology. J Gen Physiol 1942; 26: 179-193 |
| [9] | Fry WJ, Barnard JW, Fry EJ, Krumins RF, Brennan JF. Ultrasonic lesions in the mammalian central nervous system. Science 1955; 122: 517-518 |
| [10] | Chaussy Ch., Thuroff S. “Transrectal high-intensuty focused ultrasound for local treatment of prostate cancer: current role”, Arch. Esp. Urol, 2011, vol.64 (6), p. 493-506. |
| [11] | ter Haar G “Intervention and therapy”, Ultrasound Med Biol, 2000, 23 (Suppl 1), S51-S54. |
| [12] | Chapelon JY et al. “Effects of high-energy focused ultrasound on kidney tissue in the rat and the dog”, Eur Urol, 1992, vol. 22, p.147-152. |
| [13] | Uchida T et al. “Transrectal high-intensity focused ultrasound for treatment of patients with stage T1b-2n0m0 localized prostate cancer: a preliminary report”, Urology, 2002, vol.59, P.394–398. |
| [14] | Chapelon JY et al. “New piezoelectric transducers for therapeutic ultrasound”, Ultrasound Med Biol, 2002, vol. 26, p.153-159. |
| [15] | Curiel L et al. “1.5-D high intensity focused ultrasound array for non-invasive prostate cancer surgery”, IEEE Trans Ultrason Ferroelectr Freq Control, 2002, vol.49, p. 231–242. |
| [16] | Tan JS et al. “Design of focused ultrasound phased arrays for prostate treatment”, Ultrasonics Symp Proc IEEE 2, 2002, p.1247–1251. |
| [17] | Chavrier F et al. “Modeling of high intensity focused ultrasound-induced lesions in the presence of cavitation bubbles”, J Acoust Soc Am, 2000, vol.108, p. 432-440. |
| [18] | Curiel L et al. “Experimental evaluation of lesion prediction modelling in the presence of cavitation bubbles: intended for high-intensity focused ultrasound prostate treatment”, Med Biol Eng Comput, 2004, vol.42, p. 44–54. |
| [19] | Hynynen K et al. “A clinical, noninvasive, MR imaging-monitored ultrasound surgery method”, Radiographics, 1996, vol.16, p.185–195. |
| [20] | Wu T et al. “Assessment of thermal tissue ablation with MR elastography”, Magn Reson Med, 2001, vol.45. P.80-87. |
| [21] | Vaezy S et al. “Real-time visualization of high-intensity focused ultrasound treatment using ultrasound imaging”, Ultrasound Med Biol, 2001, vol.27, p.33-42. |
| [22] | Sedelaar JPM et al. “The application of three-dimensional contrast-enhanced ultrasound to measure volume of affected tissue after HIFU treatment for localized prostate cancer”, Eur Urol, 2000, vol.37, p.559-568. |
| [23] | Lu J et al. “In vitro measurement of speed of sound during coagulate tissue heating”, Ultrasonics Symp Proc IEEE 2, 1996, p.1299–1302. |
| [24] | Thüroff S et al. “High-intensity focused ultrasound and localized prostate cancer: efficacy results from the European multicentric study”, J Endourol, 2003, vol.17, p.673-677. |
| [25] | Rebillard X et al. “Treatment by HIFU of prostate cancer: survey of literature and treatment indications”, Prog Urol, 2003, vol.13, p.1428-1456. |
| [26] | Djavan B., Moul J.W., Zlotta A. et al. “PSA progression following radical prostatectomy and radiation therapy: new standards in the new Millennium”, Eur Urol., vol.43, № 1, p.12-27, 2003. |
| [27] | Hellerstedt B.A., Pienta K.J. “The current state of hormonal therapy for prostate cancer”,C.A. Cancer J. Clin., vol. 52, p.154-179, 2002. |
| [28] | Tannock IF, Osoba D, Stockler MR, et al. “Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points”, J Clin Oncol., Т.14, – p.1756–64, 1996. |
| [29] | Petrylak DP, Tangen CM, Hussain MH, et al. “Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer”, N Engl J Med., Т.351, p.1513–20, 2004. |
| [30] | Tannock IF, de Wit R, Berry WR, et al. “Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer”, N Engl J Med., T.351, p.1502–12, 2004. |
| [31] | Blana A, MuratFJ, Walter B, Thuroff S, Wieland WF, Chaussy C, et al. “First analisys of the long-term result with High-intensity focused ultrasound with localised prostate cancer”, Eur. Urol., T. 53, p. 1194 –201, 2008. |
| [32] | Uchida T, Shoji S, Nakano M, Hongo S, Nitta M, Murota A, Nagata Y. “Transrectal high-intensity focused ultrasound for the treatment of localized prostate cancer: eight-year experience”. Int J Urol., Nov. – T.16 (11), p.881–6, 2009. |
| [33] | Gelet A, Chapelon JY, Bouvier R, Rouvie‘re O, Lyonnet D, Dubernard JM “Transrectal high intensity focused ultrasound for the treatment of localised prostate cancer: factors influencing the outcome”, Eur Urol., T. 40, p. 124–129, 2001. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML