-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Tumor Therapy
p-ISSN: 2163-2189 e-ISSN: 2163-2197
2012; 1(1): 1-5
doi: 10.5923/j.ijtt.20120101.01
Primary Endocrine Therapy for Hormone Receptor Positive Breast Cancer: a Viable Treatment Alternative
Abraham A Ayantunde , Maria Gomez , Nawaz Ruhomauly, Happy Hoque
Department of Surgery Queen Mary’s Hospital Frognal Avenue Sidcup London DA14 6LT
Correspondence to: Abraham A Ayantunde , Department of Surgery Queen Mary’s Hospital Frognal Avenue Sidcup London DA14 6LT.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Endocrine therapy in hormone receptor positive breast cancer has been demonstrated to prolong disease free-survival. This study evaluates our experience with primary endocrine therapy in patients with oestrogen receptor positive (ER+) breast cancer. Patients treated with primary endocrine agents were evaluated over 4-years period. The ER and progesterone receptor status was performed using the Allred score. ER+ patients were offered primary endocrine therapy if unfit for surgery/chemotherapy, with metastases, locally advanced cancer or declined surgery. Tamoxifen was the initial first-line agent but replaced by letrozole later in the study. Clinical response was assessed using UICC criteria. 91 patients were treated with median age of 80 years (50-96). The median duration of the treatment was 20 months (2-60). The histology was ductal in 79, lobular in 8 and tubular variety in 4. The reasons for primary endocrine therapy included advanced age (25), patients unfit for surgery (29), locally advanced disease (14), metastatic disease (7) and patient declining surgery (16). 80% of the patients derived clinical benefit from the therapy, 15 patients had progressive disease (PD) and response data were not available in 3 patients. Two patients died of metastatic disease and 4 non-cancer causes. Patients with PD had poorer survival compared with those that derived CB (P-value = 0.0001). Overall 5-year survival was 93.4% and cancer specific survival was 97.7%. Primary endocrine therapy for ER+ breast cancer does produce an excellent benefit with disease control and can prolong survival. This method should be considered as a viable option in patients who do not or cannot undergo surgery/chemotherapy.
Keywords: Breast Cancer, Primary Endocrine Therapy, Oestrogen Receptor Positivity, Clinical Response, Elderly Women
1. Introduction
- The progressive increase life expectancy and the population of the elderly in the Western world will mean that an increasing number of the elderly woman will be diagnosed with breast cancer in association with their comorbid medical problems. In fact, approximately 50% of breast cancer patients are older than 65 years and 35% of patients are older than 70 years[1,2]. Primary endocrine therapy was first described in the early 1980s as an alternative to standard therapy for older women[3,4]. The use of endocrine therapy has provided meaningful advances in breast cancer treatment and prevention. Endocrine therapy has therefore become the most important treatment option for about 70% of the women with breast cancer expressing ER-alpha.Tamoxifen is an anti-oestrogen which inhibits the activity of oestrogen by competitively blocking the oestrogen receptor (ER) in the nucleus of breast cancer cells. Aromatase inhibitors like letrozole, anastrozole (non-steroidal inhibitors) and steroidal inhibitor exemestane block the conversion of androgens to oestrogens and thereby reduce oestrogen levels in tissue and plasma[5]. Tamoxifen was the most commonly used first-line primary endocrine therapy for premenopausal women with breast cancer. However, more recent data suggest that aromatase inhibitors (AIs) are a more effective choice than tamoxifen. Third generation AIs (anastrozole, letrozole, and exemestane) have been shown to have superior response rates, time to tumour progression and survival when they are used as first line treatment in postmenopausal women with advanced breast cancer in comparison with tamoxifen[6]. These newer agents are now considered the gold standard as the first-line or second-line in the endocrine therapy of oestrogen receptor positive (ER+) and/or progesterone receptor positive (PgR+) advanced breast cancer in postmenopausal women[7].The majority (70%) of breast cancers have oestrogen receptors but the percentage does vary with age. Older patients are much more likely to have cancers with oestrogen receptors (ER) and are therefore more oestrogen dependent[8]. The proportion of hormone receptor positive breast cancer and the ER content of the cancer are known to increase with the age of the patients[9,10]. The benefit of the use of chemotherapy for primary breast carcinoma is known to decrease with patient age and ER+ breast cancer are reported to be less sensitive to the use of chemotherapy than oestrogen receptor negative (ER-) tumour[4,11,12]. The use of primary endocrine therapy for breast cancer in the elderly (unselected for ER status) has been shown in a recent Cochrane Database Systematic Reviews to have comparable overall survival outcome as surgery with or without tamoxifen[13]. The use of adjuvant endocrine therapy with tamoxifen has been shown to significantly prolong disease-free survival and overall survival in postmenopausal women with early breast cancer[14].
2. Objectives
- The aim of this study was to evaluate our experience with the use of primary endocrine therapy in patients with ER+ breast cancer.
3. Methods
- The data of breast cancer patients treated with primary endocrine agents were collected over four years. ER+ patients were offered primary endocrine therapy following multidisciplinary meeting if they were unfit for surgery/chemotherapy, had metastatic disease, locally advanced cancer or those patients who declined surgery. Standard immunohistochemical staining was performed on tumour tissue obtained by core biopsy for ER and PgR. The ER and PgR status on the core biopsy specimen was performed using the Allred score[15]. The choice of initial endocrine agent was tamoxifen and this was replaced with letrozole by 2006 as the first-line agent. Patients were followed up according to the local protocol and all patients had disease assessable by UICC criteria. Clinical response was assessed and classified according to UICC criteria[16]. Complete response (CR) is disappearance of all known disease, partial response (PR) is ≥ 50% reduction in the maximum diameter of the measurable lesions, stable disease (SD) is lesion unchanged, < 50% reduction or < 25% increase in the size of measurable lesions and progressive disease (PD) is when there is more than 25% increase in the size of measurable lesions or appearance of new lesions. Objective response (OR) is CR and PR while clinical benefit (CB) is said to be achieved with a durable OR or SD for a minimum of 6 months on the therapy. Patients were followed up at 6 weekly for 3 months, 3 monthly for a year then 6 monthly for until death or for life. Clinical size of the tumour was assessed with measuring callipers and this is compared with the original size to document the clinical response according to the UICC criteria. Imaging techniques were used where indicated to ascertain the class of clinical response to the endocrine agents. A switch to a second-line agent was done with disease progression. Statistical analysis was performed using the Statistical Package for Social Sciences software version 13.0 ( Inc. Chicago, IL, USA). Descriptive statistics were performed calculating mean and median values for continuous and discrete variables respectively. Survival analyses were performed using the Kaplan-Meier survival function and Log Rank test. P-value ≤0.05 was considered statistically significant.
4. Results
- There were 91 patients with median age of 80 (50-96) years. Seventy-three percent of the patients were above 70 years of age at diagnosis. The median clinical size of the tumour at diagnosis was 3 (1-14) cm. All patients were ER+ with median Allred score of 8 (5-8) and median PgR score of 6 (0-8). Twenty-one per cent (19/91) of the cancers were screen detected and 8.8% (8/91) presented with local skin involvement (6) or fungating lesions (2). Thirty per cent (27/91) of the patients had clinically palpable ipsilateral axillary lymph nodes. The histological distribution was ductal in 79, lobular in 8 and tubular variety in 4. The reasons for decision to offer primary endocrine treatment is shown in figure 1. The first-line endocrine agents used were tamoxifen in 23, letrozole in 64 and anastrozole in 4 patients. Ten patients had their first-line drug changed to an alternative agent on account of non-response (6), PD (2) and side effects (2). The median time to change to an alternative endocrine agent was 5 (3-12) months. The median duration of treatment with endocrine agents was 20 (2-60) months and the median follow up period was 18 (2-70).
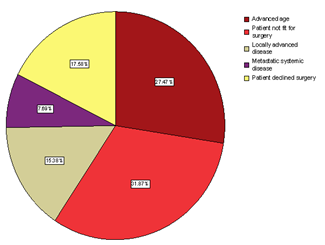 | Figure 1. Indications for primary endocrine therapy in breast cancer. |
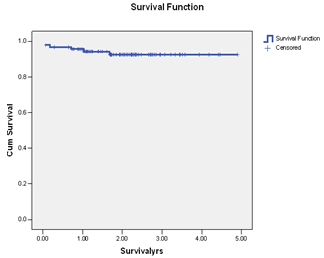 | Figure 2. Kaplan-Meier Survival function for overall survival in primary endocrine therapy. |
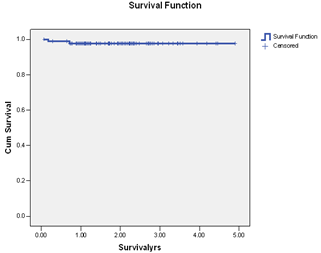 | Figure 3. Kaplan Meier survival function for breast cancer specific survival in primary endocrine therapy. |
5. Discussion
- Endocrine therapy is an important therapeutic option for patients with breast cancer and has become one of the most effective treatment strategies. It offers the potential for significant benefits in the majority of postmenopausal women with ER+ breast cancer. There has been a substantial increase and renewed interest in the use of endocrine therapy for breast cancer in the last three decades. There is an accumulated body of evidence to suggest that primary endocrine therapy is potentially superior to primary chemotherapy in postmenopausal patients with ER-positive tumours[4,11,12]. The current study clearly supports the fact that primary endocrine therapy is an alternative viable treatment option for a certain group of women with ER + breast cancer. Our results evidently confirmed that in a selected group of patients, primary endocrine therapy for breast cancer has excellent outcome and can replace other treatment options. Again, endocrine treatments are usually well tolerated by these patients, thereby increasing their acceptability as a therapeutic option in breast cancer especially in the elderly patients with one issue or the other precluding surgical intervention or the use of aggressive chemoradiotherapy. Majority of the patients in this series were elderly beyond 70 years of age with serious factors against surgery. Only 18% of our patients declined surgical intervention on personal ground. The use of tamoxifen alone to treat breast cancer has been previously investigated as an alternative to surgery in women over 70 years of age. The objective response rates (CR and PR) in phase II trial of primary tamoxifen therapy ranged from 41% to 81%, and the median time to achieve response was 3 to 5 months[17,18]. The obvious benefit of tamoxifen in adjuvant setting has generated widespread interest in the investigations comparing primary tamoxifen therapy versus immediate surgery followed by adjuvant tamoxifen treatment. This has also led to the use of primary endocrine therapy in elderly patients with ER+ cancer especially when surgery is precluded or refused. . There is a growing body of evidence to suggest that optimal endocrine therapy for hormone positive breast cancer in postmenopausal women should include an aromatase inhibitor (AI). Third generation AI letrozole has replaced tamoxifen as the first-line agent in primary endocrine therapy. Third generation AIs have been shown to have superior response rates, time to tumour progression and survival in postmenopausal women with advanced breast cancer in comparison with tamoxifen[6]. They are now considered the gold standard as the first-line or second-line in the endocrine therapy of ER + and/or PR + advanced breast cancer in postmenopausal women[7].It has been previously shown that while considering primary chemotherapy and endocrine therapies for breast cancer, it is customary and more appropriate to consider those who have derived clinical benefit (CR, PR and SD) together and not just those with OR (CR and PR). Patient who derived CB have been shown to have better overall survival compared with those with PD[19-22]. Seventy-three of our patients derived CB from endocrine agents and 15 patients developed PD. Those who derived CB did significantly better than the patients with PD but there was no difference in the overall and disease-specific survivals of patients with OR and those with SD. This study confirmed previously reported results of endocrine therapy for breast cancer by other groups[21-25]. On the basis of comparable outcome between patients with OR (CR+PR) and SD, it is therefore rational to consider them together in reporting survival for primary endocrine therapy ER+ breast cancer. Durable SD appears to be a clinically useful criterion of therapeutic remission in elderly postmenopausal women with ER+ breast cancer and the patients in this category did equally well in terms of the overall survival as those with OR.The overall survival in our patients was 93.4% while cancer-specific survival was 97.7%. This result compared favourably with those reported for any other multimodal therapy for breast cancer. Two of our patients died of metastatic disease while four died of natural causes with no obvious evidence of metastatic disease. Mathew et al[22] in a recent study of primary endocrine therapy for breast cancer also submitted that the majority of the patients were elderly and most deaths in their series were due to other causes rather than from metastatic disease. Primary endocrine therapy in the postmenopausal women with ER+ breast cancer therefore allows for definitive local control of the disease without the need to subject them to surgery, chemotherapy or radiotherapy with the potential adverse events and associated morbidities.
6. Conclusions
- The significant impact of primary endocrine therapy in postmenopausal women with ER+ breast cancer has been clearly established and does produce excellent clinical benefits with disease control and can prolong survival with avoidance of surgery or radical chemoradiotherapy. The introduction and the use of newer agents such as the third generation AIs with rapid onset and profound inhibitory activities on ER+ breast cancer, with very few agonist effects and low side effect profiles make them a viable therapeutic option in the primary endocrine setting for hormone receptor positive breast cancer in postmenopausal women who do not want to or cannot undergo surgery or any other multimodal therapy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML