-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Theoretical and Mathematical Physics
p-ISSN: 2167-6844 e-ISSN: 2167-6852
2025; 15(1): 1-3
doi:10.5923/j.ijtmp.20251501.01
Received: Jan. 16, 2025; Accepted: Feb. 5, 2025; Published: Feb. 8, 2025

On De Broglie’s Wave-Particle Theory
Fleur T. Tehrani
Professor, Department of Electrical and Computer Engineering, California State University Fullerton, Fullerton, California, USA
Correspondence to: Fleur T. Tehrani, Professor, Department of Electrical and Computer Engineering, California State University Fullerton, Fullerton, California, USA.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The purpose of this paper is to focus on De Broglie’s wave-particle duality theorem and examine some questions that can be raised based on that theorem. Those questions pertain to applications of the theorem in non-quantum large particles as well as quantum entities, and the applicability of De Broglie’s wavelength formula under different circumstances.
Keywords: Wave-Particle Duality Theorem, Intrinsic Energy, Quantum Entities
Cite this paper: Fleur T. Tehrani, On De Broglie’s Wave-Particle Theory, International Journal of Theoretical and Mathematical Physics, Vol. 15 No. 1, 2025, pp. 1-3. doi: 10.5923/j.ijtmp.20251501.01.
Article Outline
1. Introduction
- In 1923, Louis De Broglie proposed that everything manifested both wave and particle properties [1]. This new hypothesis could explain the nature of light and the manner of propagation of light and quantum entities such as electrons, that had puzzled physicists at that time. The main experiment designed to test this hypothesis was by Davisson and Germer in 1927 [2]. Since that time, many researchers have discussed the uncertainty principle of quantum mechanics that includes wave-particle theory [3]. Many physicists have suggested that the popular wavefunction formulation that is the standard problem-solving method in quantum mechanics is a mathematical tool rather than a physical entity [4,5,6,7,8]. However, the question of the meaning of the mathematics, in relation to the underlying realities of physical laws of quantum mechanics that include the wave-particle theory, has not been clearly answered to this date. More recently, some questions have been raised in regard to applicability of the wave-particle theory to light [9]. This paper examines some questions that can be raised in regard to this theorem, also in view of recent experiments.
2. Raised Questions: Is De Broglie’s Formula Based on the Intrinsic Energy Content of Mass and Can It Also Describe Energy Emissions of Quantum Entities?
2.1. De Broglie’s Formula is Intertwined with the Equation of Energy Content of Mass
- Energy of a wave is given by Planck’s equation as:
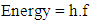 | (1) |
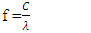 | (2) |
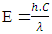 Where E is energy. Rearranging for λ:
Where E is energy. Rearranging for λ: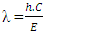 | (3) |
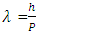 | (4) |
 | (5) |
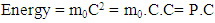 | (6) |
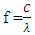 Substituting C in equation 6 by f.λ from equation 2 yields:
Substituting C in equation 6 by f.λ from equation 2 yields: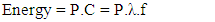 | (7) |
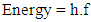 Equating equations 1 and 7 yields:
Equating equations 1 and 7 yields: | (8) |
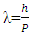 which is De Broglie’s equation 4. Based on this equation, the following questions can be raised.
which is De Broglie’s equation 4. Based on this equation, the following questions can be raised.2.2. Are Non-Quantum Particles Associated with Waves in Motion as Proposed by the Wave-Particle Theory?
- The validity of equation 5 which is considered as a law, has been confirmed by many experiments. Equation 5 indicates that mass is convertible to energy, which further indicates that mass and energy may be considered as fundamentally the same despite being considered as different physical entities. This equation shows the intrinsic energy content of a particle as given by m0C2.If one accepts that non-quantum particles are associated with energy waves in motion at a wavelength λ as given by De Broglie’s formula (equation 4), where does that energy field of the particle come from? The proposed energy waves of large non-quantum particles have not been confirmed experimentally. There is no experimental report showing the proposed energy waves of even small, microscopic, non-quantum particles. It has been explained that since for particles that are not at the atomic and sub-atomic levels, λ is very small, it cannot be detected and measured experimentally. However, this does not explain/prove that non-quantum particles are associated with energy waves in motion.
2.3. Are Quantum Particles Associated with Energy Waves with Wavelengths Given by De Broglie’s Formula, Equation 4?
- Davisson and Germer reported that the wavelength of electron beams energized by thermionic emission, was measured as predicted by De Broglie’s formula (equation 4). In the reported experiments, electrons’ masses were not converted to energy. The experimental results were used to prove the wave nature of electrons, and that matter manifests both particle and wave characteristics [2]. According to De Broglie’s theory, electrons that are bound in the atomic structure are associated with a wave where the wavelength is given by equation 4. In the experiments reported by Davisson and Germer [2], the electron beams were made of free electrons energized by thermionic emission and further accelerated by an external electric field. The propagation pattern of those beams was found to be a wave pattern. The wavelength was calculated in accordance with De Broglie’s formula, equation 4, where momentum, P, was found from the kinetic energy of electrons provided by the external electric field. One could argue that the observed calculated wavelength in those experiments was not necessarily associated with the wavelength of electrons bound in an atomic structure. The question arises that if electrons are energized in other types of experiments, will the wavelengths of energies given off by those electrons be in accordance with equation 4?Electrons have been known to give off energy under certain circumstances. When energized electrons go back to their initial quantized levels of energy, they give off the difference in the energy levels. In that process, the frequency of the electron’s given off energy wave is directly proportional to the energy difference between the two energy levels and is given by:
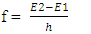 | (9) |
3. Summary
- According to some reported experiments [2], energy as well as particles at subatomic levels are observed to propagate in waves. This is plausible; sound propagates through the substance as energy waves and those waves may contain small particles [12]. De Broglie’s formula (equation 4) can be derived from equations 1 and 5 and is intertwined with the intrinsic energy content of mass. The following questions can be raised in regard to applicability of De Broglie’s formula, equation 4, under different circumstances: 1. If a non-quantum particle is associated with an energy wave, a phenomenon that has not been confirmed experimentally, where does that field of energy come from and how this phenomenon can be verified experimentally.2. Davisson and Germer experiment [2] was focused on beams of free electrons energized by thermionic emission and accelerated by an external electric field. Davisson and Germer did not calculate the wavelength of any electron wave for any electron bound in an atomic structure and they used the external kinetic energy provided to electrons to calculate momentum, P, in equation 4. At the quantum levels, mass and energy are quantized and quantum entities are assumed to give off energy to keep their energies at allowed levels. In some experiments on quantum entities such as experiments on excited electrons giving off energy in moving from one level of energy to a lower level, or when free electrons are energized in cyclotrons, the applicability of equation 4 has not been reported and may need to be addressed.The above questions may deserve to be addressed to determine the scope of applicability of equation 4 and the wave-particle duality theorem under different circumstances.
Data Availability
- Data generated or analyzed during this study are available from the corresponding author upon reasonable request.
Author Information
- Author ORCID: Fleur Tehrani: https://orcid.org/0000-0001-8621-7697Author contributionsWriting, Review and Editing: F.T. TehraniCompeting interestsThe author declares there are no competing interests
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML