Walid Gewily
Department of Chemistry, Damietta Faculty of Science, Egypt
Correspondence to: Walid Gewily , Department of Chemistry, Damietta Faculty of Science, Egypt.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Since neutrons and protons are the main components of the atomic nucleus and studying their nature helps in understanding the nature of the atomic nucleus and its properties, this study has done to reveal some of the properties of neutrons depending upon the postulated properties of electron and proton in the previous study (photon as a basic unit), in this study; it was postulated that electron and proton are the main components of the neutron, based on this postulation some basic properties of neutron like structure, mass, lifetime, beta decay, and magnetic moment have been deduced and also the nature of neutrino and anti-neutrino has been illustrated as a result of studying beta decay.
Keywords:
Neutron structure, Neutron mass, Free neutron lifetime, Beta-Decay, Neutrino, Anti-neutrino, Proton magnetic moment, Electron magnetic moment, Neutron magnetic moment, Continuous energy spectrum of beta particles
Cite this paper: Walid Gewily , Neutron as a Consequence of “Photon as a Basic Unit”, International Journal of Theoretical and Mathematical Physics, Vol. 11 No. 3, 2021, pp. 99-105. doi: 10.5923/j.ijtmp.20211103.02.
1. Introduction
Rutherford In 1920 postulated that the nucleus consists of neutral particles and positive protons then he suggested that this neutral particle is formed from a proton and an electron combined in some way because beta radiation consists of electrons emitted from the nucleus [1]. In 1932 James Chadwick discovered the neutron [2] which is the neutral particle within the nucleus which was described by Rutherford. The mass of the free neutron was reported to be 939.565413±0.000006meV/c2 [3] with a mean square radius of about 0.8×10−15 m [4], the free neutron is unstable and undergoes a process called beta decay which is a type of radioactive decay at which the free neutron decays into a proton, electron, and antineutrino with a mean lifetime of (885.7±0.8 s) [3]. Beta-decay is a process at which a Beta particle (electron or positron) is emitted from the atomic nucleus; it can be classified into 3 types either by emission of an electron and an anti-neutrino, a positron and a neutrino, or by the capture of an electron with the emission of neutrino [5]. Hughes and Burgy In 1949 reported that the angular distribution of the reflected neutrons from a ferromagnetic mirror was consistent with spin 1/2 [6]. Neutron’s magnetic moment value was measured by Luis Alvarez and Felix Bloch in 1940 [7] to be −1.93(2) μN which indicates that it has a substructure.In the previous study (photon as a basic unit) [8]; a model for photon was suggested and it was postulated to be the basic component of subatomic particles with a sperm structure composed from three parts: head, separator plus the spiral tail with a mass 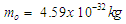 and length
and length 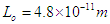 , then it was suggested that electron and positron are formed from photons in a tree form structure with one condensed spiral tail and branches of photons heads with radius
, then it was suggested that electron and positron are formed from photons in a tree form structure with one condensed spiral tail and branches of photons heads with radius 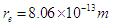 , also proton was suggested to be formed from positrons in a tree form structure with one condensed spiral tail and branches of positrons’ heads with radius
, also proton was suggested to be formed from positrons in a tree form structure with one condensed spiral tail and branches of positrons’ heads with radius 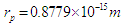 , then electron was deduced to has 2 states; bounded electron with mass
, then electron was deduced to has 2 states; bounded electron with mass 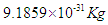 which can be converted into free electron with mass
which can be converted into free electron with mass 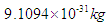 by losing one photon’s head with a mass
by losing one photon’s head with a mass 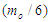 , the same with proton there is a bounded proton with mass
, the same with proton there is a bounded proton with mass 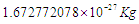 which can be converted into a free proton with mass
which can be converted into a free proton with mass 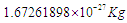 by losing one positron’s head
by losing one positron’s head 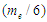 , then charge was interpreted to be the time taken by a particle to pass a certain point, and the direction motion of the spiral tail for the particle is responsible for charge sign, also a formula for spin property for subatomic particles was deduced to be
, then charge was interpreted to be the time taken by a particle to pass a certain point, and the direction motion of the spiral tail for the particle is responsible for charge sign, also a formula for spin property for subatomic particles was deduced to be 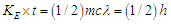 where
where 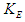 is the spin kinetic energy and
is the spin kinetic energy and 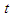 is the time of one spin.This study was done to theoretically explain and deduce some of the neutron's properties like structure, mass, beta decay, half lifetime, and magnetic moment.
is the time of one spin.This study was done to theoretically explain and deduce some of the neutron's properties like structure, mass, beta decay, half lifetime, and magnetic moment.
2. Neutron Structure
According to the quark model for hadrons, the neutron is composed of one up quark with a charge +2/3 e and two down quarks with a charge −1/3 e [9]. Based on the mentioned properties of neutron and the postulated structures for proton and electron in the previous study [8]; another point of view will be introduced for neutron structure. The neutron is postulated to be formed from the interaction and complete unification between the 2 opposite phases’ tales of electron and proton canceling the charge effect of each other resulting in a neutral particle with 3 additional postulated criteria: 1-electron’s radius will be compressed to equal proton’s radius. 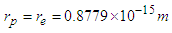 2-the length
2-the length 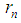 of the tail connecting the centers of proton and electron will be compressed to be.
of the tail connecting the centers of proton and electron will be compressed to be.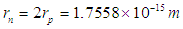 3-the proton and the electron within the neutron are in free form.The resulted structure can be postulated to be
3-the proton and the electron within the neutron are in free form.The resulted structure can be postulated to be | Figure 1. an approximation for neutron structure |
3. Neutron Mass
Neutron mass can be measured by the subtraction of proton mass from deuteron mass, which gives also the binding energy of deuterium; which can be obtained by measuring the energy of gamma-ray emitted when a proton captures a neutron forming a deuteron, in 1948 Bell and Elliot measured the energy of this gamma-ray by X-ray diffraction techniques and the neutron mass is given by Greene, et al. to be 1.008644904(14) Da [10] which equals 1.674894241×10-27 Kgm.In this study according to the suggested structure for neutron; its mass  can be calculated by the summation of the masses of proton
can be calculated by the summation of the masses of proton  , electron
, electron 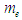 , and the mass
, and the mass 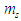 equivalent to the energy
equivalent to the energy 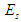 stored in the compressed tail between proton and electron which can be calculated by coulomb’s law;
stored in the compressed tail between proton and electron which can be calculated by coulomb’s law;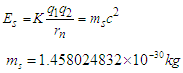 The total mass of the neutron
The total mass of the neutron 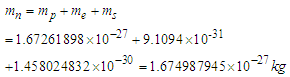
4. Free Neutron Lifetime
The exact value of the mean lifetime for the neutron is still uncertain; this problem is called the “neutron lifetime puzzle”. This is due to conflicting results from experiments e.g. lifetime value from the magnetic trap with permanent magnets [11] was measured to be 878.2±1.9s. In the MAMBO II experiment [12] the result was obtained to be 880.7±1.8s, and the results of the experiments with room temperature fomblin were reported to be 882.5±2.1s [13] and 881.6±2.1s [14] then another experiment gave the value for neutron lifetime to be 880.2±1.2s [15]. The analyses of neutron lifetime value from different experiments are with asymmetry data from Particle Data Group 885.7±0.8 s [3].In order to solve this enigma; we have to configure the decay process for the free neutron. According to the current study; the gained energies stored in the compressed electron and the connecting tail between proton and electron within the free neutron make it unstable and tend to decay by separating the electron from the proton. The kinetic energy 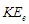 required for the electron to escape from the proton has to equal the gravitational energy of the mass
required for the electron to escape from the proton has to equal the gravitational energy of the mass 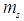 stored in the connecting tail;
stored in the connecting tail;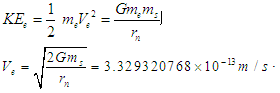
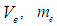 and
and 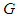 are the electron’s escaping velocity, electron mass, and universal gravitational constant respectively.So it can be concluded that free neutron decay depends on electron escaping velocity
are the electron’s escaping velocity, electron mass, and universal gravitational constant respectively.So it can be concluded that free neutron decay depends on electron escaping velocity 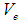 and the distance
and the distance 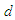 which the electron has to pass, this distance has to be greater than the longest available distance between a nucleus and its outer shell atomic electron e.g. if an electron escapes with a velocity
which the electron has to pass, this distance has to be greater than the longest available distance between a nucleus and its outer shell atomic electron e.g. if an electron escapes with a velocity 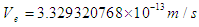 and passes a distance
and passes a distance 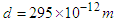 , the time
, the time 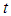 taken will be
taken will be 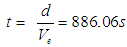 ; which is the time required for a free neutron to decay.So we can conclude that there is no solid number for the neutron lifetime but it depends upon the distance (d) which the electron within the neutron has to pass and overcome the attraction force with the proton and the decay process occurs.
; which is the time required for a free neutron to decay.So we can conclude that there is no solid number for the neutron lifetime but it depends upon the distance (d) which the electron within the neutron has to pass and overcome the attraction force with the proton and the decay process occurs.
5. Beta-Decay
The next types of beta decay can be illustrated based on the suggested models of electron, proton, and neutron.
5.1. Emission of an Electron 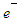 and Anti-Neutrino
and Anti-Neutrino 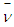
In this type of beta decay neutron is converted into a proton by emission of an electron and an antineutrino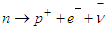 | (1) |
This type of decay can be illustrated by the next figure 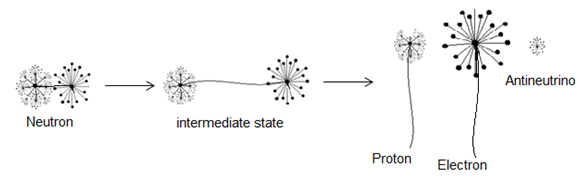 | Figure 2. an approximation for neutron decay |
The gravitational force 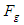 between electron and proton within the neutron
between electron and proton within the neutron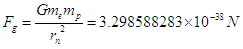 The gravitational force
The gravitational force 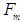 between one positron head with a mass
between one positron head with a mass 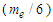 within a proton and the center of the proton
within a proton and the center of the proton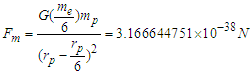
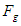 is the minimum force required by an electron to escape from the gravitational force of a proton within the neutron. According to the third law of newton; the proton will counter this effect with the same amount of force which is sufficient to overcome the force
is the minimum force required by an electron to escape from the gravitational force of a proton within the neutron. According to the third law of newton; the proton will counter this effect with the same amount of force which is sufficient to overcome the force 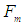 which results in releasing a mass
which results in releasing a mass 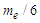 (one positron head) from the proton, the difference between the two forces
(one positron head) from the proton, the difference between the two forces 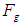 and
and 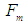 will be used in proton recoil.The neutron decay equation can be written as
will be used in proton recoil.The neutron decay equation can be written as 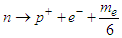 | (2) |
By equating equations (1) and (2); the antineutrino 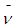 can be considered to be one positron head.
can be considered to be one positron head.
5.2. Emission of a Positron 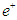 and Neutrino
and Neutrino 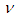
In this type of beta decay, a proton is converted into a neutron by the emission of a positron and a neutrino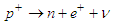 | (3) |
This type of decay can be illustrated by the next figure 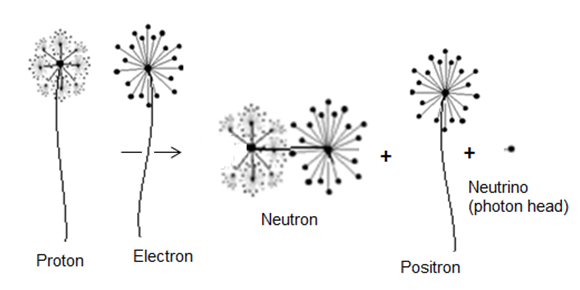 | Figure 3. an approximation for conversion of a proton into a neutron |
According to the current study; conversion of a proton into a neutron requires an electron. since the proton is formed from positrons in a tree form structure; so if the proton loses a positron the proton will compensate this loss in mass by attracting an equivalent mass from the surrounding medium which will be a bounded electron to form a neutron, according to role number 3 in neutron structure section; the bounded electron within the new neutron will be converted into free-electron by losing one photon head with mass 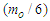 .And the decay equation can be written as
.And the decay equation can be written as 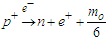 | (4) |
In this equation, electric charge conservation was obeyed where the left side of the equation resulted in a positively charged atom. By equating equations (3) and (4); the neutrino 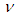 can be considered as one photon head
can be considered as one photon head 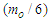 .
.
5.3. Capture of an Electron with the Emission of a Neutrino
In this type of beta decay, a proton is converted into a neutron by attracting an electron from the inner shell and emitting a neutrino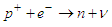 | (5) |
This type of decay can be illustrated by the next figure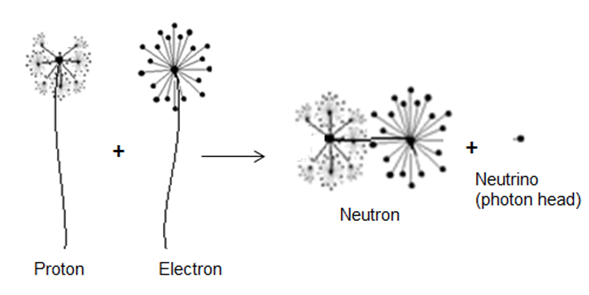 | Figure 4. An approximation for the Capture of an electron with the emission of a neutrino |
Based on this study, in proton-rich nuclei; the free proton will capture bounded electrons from a near electron shell to form a neutron by releasing photon head with a mass 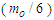 from the electron to be in free form within the nucleus
from the electron to be in free form within the nucleus 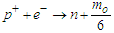 | (6) |
By equating equations (5) and (6); the neutrino 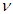 can be considered as one photon head
can be considered as one photon head 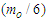 . It can be deduced that in the 3 previous beta decay types 2 byproducts have been resulted (
. It can be deduced that in the 3 previous beta decay types 2 byproducts have been resulted (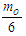 and
and 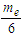 ) which can be used to explain the inverse beta decay e.g. in KamLAND experiment [16]; neutrinos were detected by inverse beta decay with threshold energy of about 2.6 MeV
) which can be used to explain the inverse beta decay e.g. in KamLAND experiment [16]; neutrinos were detected by inverse beta decay with threshold energy of about 2.6 MeV 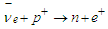 | (7) |
Since 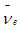 represents positron head
represents positron head  , upon collision in an energy level of about 2.6 MeV which is greater than the Coulomb force between one positron head and the center of the proton; the proton will counter this action by repelling one positron from that proton, hence to compensate the loss in proton mass it will attract a bounded electron from the surrounding medium converting it into a free electron by losing photon head
, upon collision in an energy level of about 2.6 MeV which is greater than the Coulomb force between one positron head and the center of the proton; the proton will counter this action by repelling one positron from that proton, hence to compensate the loss in proton mass it will attract a bounded electron from the surrounding medium converting it into a free electron by losing photon head  and forming a neutron.
and forming a neutron.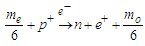 | (8) |
Also in this equation, electric charge conservation was obeyed where the left side of the equation resulted in a positively charged atom.
6. Proton and Electron Magnetic Moments
The recommended values for the magnetic moment of the proton and electron according to CODATA [17] are 1.410 606 797 36 x 10-26 J T-1 and -9.284 764 7043 x 10-24 J T-1 respectively. According to the current study; in order to derive the values of the magnetic moment for electron and proton we have to follow the assumption that electron and proton are not point-like particles and have a definite structure [8], so during their orbital motion they form a cone structure with the orbiting center with an area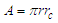 | (9) |
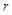 is the subatomic particle radius and
is the subatomic particle radius and 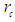 is the lateral radius of the formed cone, as shown in the next figure.
is the lateral radius of the formed cone, as shown in the next figure. 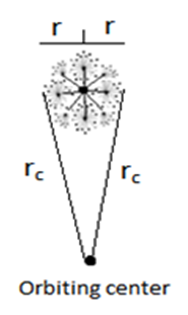 | Figure 5. a cone structure formed by the orbital motion of a subatomic particle |
The rate of change of angular momentum of a subatomic particle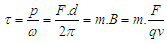 | (10) |
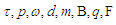 and
and 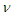 are torque, power, angular velocity, linear distance, magnetic moment, external magnetic field, charge, force, and tangential velocity respectively.It was postulated that charge
are torque, power, angular velocity, linear distance, magnetic moment, external magnetic field, charge, force, and tangential velocity respectively.It was postulated that charge 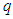 is the time taken by a particle to pass a certain point [8] and distance=time X velocity
is the time taken by a particle to pass a certain point [8] and distance=time X velocity 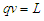 | (11) |
from equations (10), (11) magnetic moment 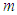 can be deduced to be
can be deduced to be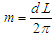 | (12) |
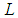 will be postulated to be a length in meter unit of the circumference of the orbital motionSo the lateral radius of the formed cone
will be postulated to be a length in meter unit of the circumference of the orbital motionSo the lateral radius of the formed cone 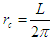
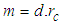 | (13) |
By equating equations (9) and (13)If 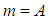 so
so 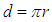
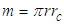 | (14) |
According to this equation, the magnetic moment can be identified to be the area of the cone made by the subatomic particle during its orbital motion around the orbiting center.Lateral radius can be calculated by calculating orbital kinetic energy and the velocity of the particle. The formula for spin kinetic energy 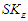 for stable particles [8] with charge
for stable particles [8] with charge 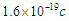 is
is 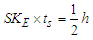 | (15) |
time of one spin 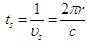
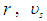 and
and 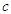 are the particle radius, frequency of spin kinetic energy, and light velocity respectively.
are the particle radius, frequency of spin kinetic energy, and light velocity respectively.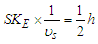 | (16) |
According to plank’s black body radiation formula 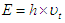 where
where 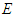 ,
, 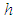 and
and 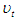 are energy, plank's constant, and frequency respectively. Multiply both sides of equation (16) by
are energy, plank's constant, and frequency respectively. Multiply both sides of equation (16) by 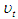 .
.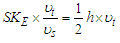 | (17) |
For electron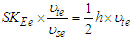 | (18) |
for proton 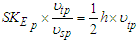 | (19) |
since electron and proton and most stable particles have the same charge and frequency 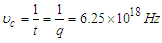 .To verify that concept for two different particles like electron and proton; equations (18, 19) have to be multiplied by the reciprocal
.To verify that concept for two different particles like electron and proton; equations (18, 19) have to be multiplied by the reciprocal 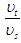 of the other particle, which will result in the orbital kinetic energy formula
of the other particle, which will result in the orbital kinetic energy formula 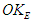 .Orbital kinetic energy for electron
.Orbital kinetic energy for electron 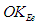
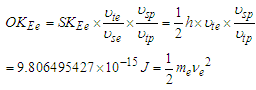 | (20) |
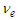 is the tangential velocity of the electron
is the tangential velocity of the electron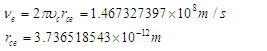 | (21) |
Electron magnetic moment 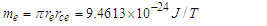 | (22) |
Orbital kinetic energy for proton 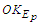
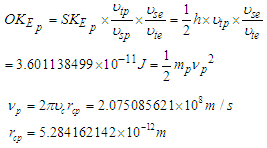 | (23) |
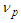 is the tangential velocity of the protonProton magnetic moment
is the tangential velocity of the protonProton magnetic moment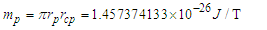 | (24) |
The deduced values for the derived magnetic moments of electron and proton are close to the values stated in CODATA.
7. Neutron Magnetic Moment
According to CODATA, the magnetic moment value of neutron is (-9.662 3651 x 10-27 J T-1) [17], the magnetic moment value for neutron was puzzling until the development of the quark model which stated that neutron is composed from three quarks and its magnetic moment is the summation of these three particles [9].In this study another point of view will be introduced; neutron structure is a problem between 2 particles (electron and proton) so its orbital motion lateral radius 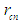 can be concluded to be a reduced radius
can be concluded to be a reduced radius 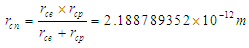 According to structure (1); neutron root mean square radius (r.m.s)
According to structure (1); neutron root mean square radius (r.m.s) 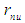
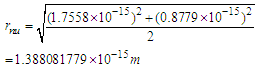 Neutron magnetic moment
Neutron magnetic moment 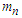
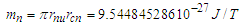 | (25) |
And this value is close to the value stated in CODATA 2018.This rule could be applied for other subatomic particles e.g. muon.
8. Muon Magnetic Moment
Muon is a subatomic particle similar to the electron in charge and a spin with a mass 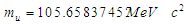 [3].And magnetic moment (-4.490 448 30 x 10-26 J T-1 ) according to CODATA [17]. Its radius can be calculated as the same as electron radius in the previous study [8] as follows;
[3].And magnetic moment (-4.490 448 30 x 10-26 J T-1 ) according to CODATA [17]. Its radius can be calculated as the same as electron radius in the previous study [8] as follows; 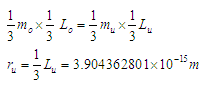
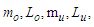 and
and 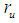 are photon mass, photon length, muon mass, muon length, and muon radius respectively.Muon orbital kinetic energy
are photon mass, photon length, muon mass, muon length, and muon radius respectively.Muon orbital kinetic energy 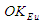
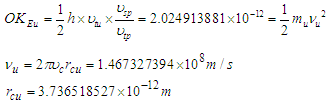 | (26) |
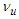 is the tangential velocity of the electronMuon magnetic moment
is the tangential velocity of the electronMuon magnetic moment 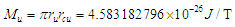 | (27) |
Magnetic moment sign (positive or negative) depends upon the direction motion of the orbital kinetic energy of the subatomic particle (clockwise or anticlockwise).
9. Continuous Energy Spectrum of Beta Particles
It was postulated in the first decade of the twentieth century that beta particles emitted in radioactive decay were mono-energetic after that it was discovered to have continuous energy spectrum which was explained by Pauli’s hypothesis [18] by the presence of a neutral and zero mass particle that is emitted with the beta particle and is sharing it in momentum and energy. Then Fermi formulated a theory of beta decay on the basis of this idea [19].In this study another point of view for the continuous energy spectrum of the beta particles will be introduced:Since the continuous energy spectrum of beta particles is an indication for the different velocities of the emitted beta particles during beta decay so the continuous energy spectrum can be explained by showing the factors which control the velocities of the electrons during beta decay. It was mentioned in the neutron structure section that electron radius 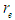 within neutron was compressed from
within neutron was compressed from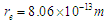 to
to 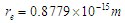 Using equations (21), (22)
Using equations (21), (22)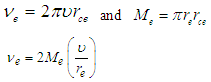 | (28) |
In the case of the constant magnetic moment of the electron, it can be deduced that the velocity 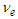 of the escaped electron in beta decay will not be constant and will depend on the ratio of the electron frequency to its radius where the electron tends to retain its original radius during escaping, as a result, the electrons will have different velocities and continuous energy spectrum.
of the escaped electron in beta decay will not be constant and will depend on the ratio of the electron frequency to its radius where the electron tends to retain its original radius during escaping, as a result, the electrons will have different velocities and continuous energy spectrum.
10. Conclusions
Based on the previous study (photon as a basic unit), some postulations about neutron's intrinsic fundamental properties like structure, mass, radius, lifetime, beta decay, and the magnetic moment have been introduced. Neutron was postulated to be formed from the interaction and complete unification between the 2 opposite phases' tales of electron and proton, electron's radius was compressed to equal proton's radius, neutron mas was calculated from the summation of the masses of proton, electron, and the mass equivalent to the energy stored in the compressed tail between proton and electron to be 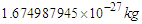 . Due to the gained energies within the neutron which are stored in the compressed electron and the connecting tail between proton and electron; the free neutron became unstable and tends to decay, its decay depends on the electron's escaping velocity and the distance which the electron has to pass for escaping.Three types of beta decay were studied and resulted in postulation that neutrino and anti-neutrino are one photon head and one positron head respectively.The magnetic moment was deduced to be the area of the cone made by the subatomic particle during its orbital motion around the orbiting center, and according to this postulation magnetic moment values for electron, proton, neutron, and muon were deduced to be
. Due to the gained energies within the neutron which are stored in the compressed electron and the connecting tail between proton and electron; the free neutron became unstable and tends to decay, its decay depends on the electron's escaping velocity and the distance which the electron has to pass for escaping.Three types of beta decay were studied and resulted in postulation that neutrino and anti-neutrino are one photon head and one positron head respectively.The magnetic moment was deduced to be the area of the cone made by the subatomic particle during its orbital motion around the orbiting center, and according to this postulation magnetic moment values for electron, proton, neutron, and muon were deduced to be 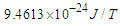 ,
,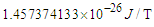 ,
,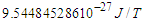 , and
, and 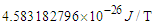 respectively. The velocity of the escaped electron in beta decay was deduced to not be a constant and depends on the ratio of the emitted electron frequency to its radius during escaping resulting in a continuous energy spectrum for the emitted beta particles.Based on this study and the previous study (photon as a basic unit); the area for a new nuclear model study will be opened.
respectively. The velocity of the escaped electron in beta decay was deduced to not be a constant and depends on the ratio of the emitted electron frequency to its radius during escaping resulting in a continuous energy spectrum for the emitted beta particles.Based on this study and the previous study (photon as a basic unit); the area for a new nuclear model study will be opened.
References
| [1] | Rutherford, E. (1920). "Nuclear Constitution of Atoms". Proceedings of the Royal Society A. 97 (686): 374–400. |
| [2] | Chadwick, James (1932). "Possible Existence of a Neutron". Nature. 129 (3252): 312. |
| [3] | C.Patrignani et al. (Particle Data Group). "Review of Particle Physics". Journal of Chin. Phys. C, 40, 100001 (2016). |
| [4] | Povh, B.; Rith, K.; Scholz, C.; Zetsche, F. (2002). "Particles and Nuclei: An Introduction to the Physical Concepts" Berlin Springer-Verlag. p. 73. |
| [5] | N. Cottingham, D. A. Greenwood. "An introduction to nuclear physics Second edition" 2001. Cambridge University Press. p. 163. |
| [6] | Hughes, D.J.; Burgy, M.T. (1949). "Reflection and polarization of neutrons by magnetized mirrors". Physical Review. 76 (9): 1413–1414. |
| [7] | Alvarez, L.W; Bloch, F. (1940). "A quantitative determination of the neutron magnetic moment in absolute nuclear magnetons". Physical Review. 57 (2): 111–122. |
| [8] | Walid gewily. (2018). "Photon as a basic unit". International journal of theoretical and mathematical physics. 8(4): 79-88. |
| [9] | Gell, Y.; Lichtenberg, D.B. (1969). "Quark model and the magnetic moments of proton and neutron". Il Nuovo Cimento A. Series 10. 61 (1): 27–40. |
| [10] | Greene, GL; et al. (1986). "New determination of the deuteron binding energy and the neutron mass". Physical Review Letters. 56 (8): 819–822. |
| [11] | V.F. Ezhov, B. A. Bazarov, P. Geltenbort et. al. Tech. Phys. Lett., Vol. 27, No. 12, 2001. pp. 1055-1057. |
| [12] | A. Pichlmaier, V. Varlamov, K. Schreckenbach, P. Geltenbort. Phys. Lett. B, Vol. 693, 2010. pp. 221-226. |
| [13] | A. Steyerl, J. M. Pendlebury, C. Kaufman, et al. Phys. Rev. C, Vol. 85, No. 065503, 2012. pp. 1-14. |
| [14] | S. S. Arzumanov, L. N. Bondarenko, V. I. Morozov et. al. JETP Lett., Vol. 95, No. 5, 2012. pp. 224-228. |
| [15] | S. Arzumanov, L. Bondarenko, S. Chernyavsky et. al. Phys. Lett. B, Vol. 745, 2015. pp. 79-89. |
| [16] | K. Eguchi et al., "First results from KamLAND: Evidence for reactor anti-neutrino disappearance," Phys. Rev. Lett., vol. 90, p. 021802, 2003. |
| [17] | CODATA RECOMMENDED VALUES OF THE FUNDAMENTAL PHYSICAL CONSTANTS: 2018 NIST SP 961 (May 2019). |
| [18] | W. Pauli letter to nuclear physicists in Tuebingen, Germany, 1930. |
| [19] | E. Fermi, Il Nuovo Cimento, Volume 9, 1 (1934). |


 and length
and length 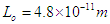 , then it was suggested that electron and positron are formed from photons in a tree form structure with one condensed spiral tail and branches of photons heads with radius
, then it was suggested that electron and positron are formed from photons in a tree form structure with one condensed spiral tail and branches of photons heads with radius 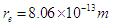 , also proton was suggested to be formed from positrons in a tree form structure with one condensed spiral tail and branches of positrons’ heads with radius
, also proton was suggested to be formed from positrons in a tree form structure with one condensed spiral tail and branches of positrons’ heads with radius 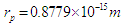 , then electron was deduced to has 2 states; bounded electron with mass
, then electron was deduced to has 2 states; bounded electron with mass 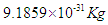 which can be converted into free electron with mass
which can be converted into free electron with mass 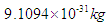 by losing one photon’s head with a mass
by losing one photon’s head with a mass 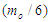 , the same with proton there is a bounded proton with mass
, the same with proton there is a bounded proton with mass 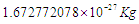 which can be converted into a free proton with mass
which can be converted into a free proton with mass 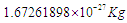 by losing one positron’s head
by losing one positron’s head 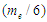 , then charge was interpreted to be the time taken by a particle to pass a certain point, and the direction motion of the spiral tail for the particle is responsible for charge sign, also a formula for spin property for subatomic particles was deduced to be
, then charge was interpreted to be the time taken by a particle to pass a certain point, and the direction motion of the spiral tail for the particle is responsible for charge sign, also a formula for spin property for subatomic particles was deduced to be 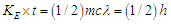 where
where 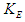 is the spin kinetic energy and
is the spin kinetic energy and 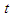 is the time of one spin.This study was done to theoretically explain and deduce some of the neutron's properties like structure, mass, beta decay, half lifetime, and magnetic moment.
is the time of one spin.This study was done to theoretically explain and deduce some of the neutron's properties like structure, mass, beta decay, half lifetime, and magnetic moment.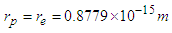 2-the length
2-the length 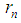 of the tail connecting the centers of proton and electron will be compressed to be.
of the tail connecting the centers of proton and electron will be compressed to be.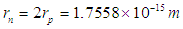 3-the proton and the electron within the neutron are in free form.The resulted structure can be postulated to be
3-the proton and the electron within the neutron are in free form.The resulted structure can be postulated to be
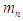 can be calculated by the summation of the masses of proton
can be calculated by the summation of the masses of proton 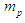 , electron
, electron 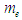 , and the mass
, and the mass 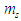 equivalent to the energy
equivalent to the energy 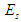 stored in the compressed tail between proton and electron which can be calculated by coulomb’s law;
stored in the compressed tail between proton and electron which can be calculated by coulomb’s law;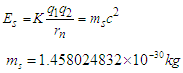 The total mass of the neutron
The total mass of the neutron 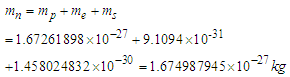
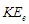 required for the electron to escape from the proton has to equal the gravitational energy of the mass
required for the electron to escape from the proton has to equal the gravitational energy of the mass 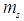 stored in the connecting tail;
stored in the connecting tail;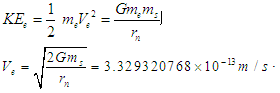
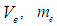 and
and 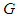 are the electron’s escaping velocity, electron mass, and universal gravitational constant respectively.So it can be concluded that free neutron decay depends on electron escaping velocity
are the electron’s escaping velocity, electron mass, and universal gravitational constant respectively.So it can be concluded that free neutron decay depends on electron escaping velocity 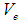 and the distance
and the distance 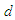 which the electron has to pass, this distance has to be greater than the longest available distance between a nucleus and its outer shell atomic electron e.g. if an electron escapes with a velocity
which the electron has to pass, this distance has to be greater than the longest available distance between a nucleus and its outer shell atomic electron e.g. if an electron escapes with a velocity 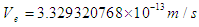 and passes a distance
and passes a distance 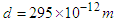 , the time
, the time 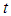 taken will be
taken will be 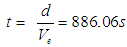 ; which is the time required for a free neutron to decay.So we can conclude that there is no solid number for the neutron lifetime but it depends upon the distance (d) which the electron within the neutron has to pass and overcome the attraction force with the proton and the decay process occurs.
; which is the time required for a free neutron to decay.So we can conclude that there is no solid number for the neutron lifetime but it depends upon the distance (d) which the electron within the neutron has to pass and overcome the attraction force with the proton and the decay process occurs. 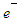 and Anti-Neutrino
and Anti-Neutrino 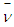
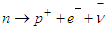

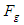 between electron and proton within the neutron
between electron and proton within the neutron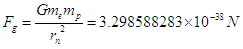 The gravitational force
The gravitational force 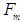 between one positron head with a mass
between one positron head with a mass 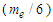 within a proton and the center of the proton
within a proton and the center of the proton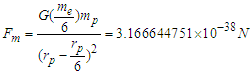
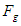 is the minimum force required by an electron to escape from the gravitational force of a proton within the neutron. According to the third law of newton; the proton will counter this effect with the same amount of force which is sufficient to overcome the force
is the minimum force required by an electron to escape from the gravitational force of a proton within the neutron. According to the third law of newton; the proton will counter this effect with the same amount of force which is sufficient to overcome the force 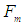 which results in releasing a mass
which results in releasing a mass 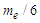 (one positron head) from the proton, the difference between the two forces
(one positron head) from the proton, the difference between the two forces 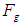 and
and 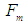 will be used in proton recoil.The neutron decay equation can be written as
will be used in proton recoil.The neutron decay equation can be written as 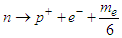
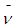 can be considered to be one positron head.
can be considered to be one positron head. 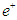 and Neutrino
and Neutrino 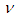
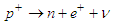

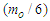 .And the decay equation can be written as
.And the decay equation can be written as 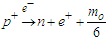
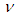 can be considered as one photon head
can be considered as one photon head 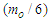 .
.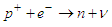

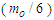 from the electron to be in free form within the nucleus
from the electron to be in free form within the nucleus 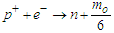
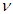 can be considered as one photon head
can be considered as one photon head 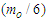 . It can be deduced that in the 3 previous beta decay types 2 byproducts have been resulted (
. It can be deduced that in the 3 previous beta decay types 2 byproducts have been resulted (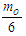 and
and 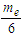 ) which can be used to explain the inverse beta decay e.g. in KamLAND experiment [16]; neutrinos were detected by inverse beta decay with threshold energy of about 2.6 MeV
) which can be used to explain the inverse beta decay e.g. in KamLAND experiment [16]; neutrinos were detected by inverse beta decay with threshold energy of about 2.6 MeV 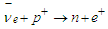
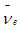 represents positron head
represents positron head 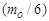 , upon collision in an energy level of about 2.6 MeV which is greater than the Coulomb force between one positron head and the center of the proton; the proton will counter this action by repelling one positron from that proton, hence to compensate the loss in proton mass it will attract a bounded electron from the surrounding medium converting it into a free electron by losing photon head
, upon collision in an energy level of about 2.6 MeV which is greater than the Coulomb force between one positron head and the center of the proton; the proton will counter this action by repelling one positron from that proton, hence to compensate the loss in proton mass it will attract a bounded electron from the surrounding medium converting it into a free electron by losing photon head 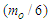 and forming a neutron.
and forming a neutron.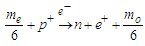
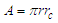
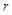 is the subatomic particle radius and
is the subatomic particle radius and 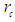 is the lateral radius of the formed cone, as shown in the next figure.
is the lateral radius of the formed cone, as shown in the next figure. 
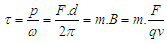
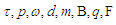 and
and 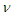 are torque, power, angular velocity, linear distance, magnetic moment, external magnetic field, charge, force, and tangential velocity respectively.It was postulated that charge
are torque, power, angular velocity, linear distance, magnetic moment, external magnetic field, charge, force, and tangential velocity respectively.It was postulated that charge 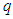 is the time taken by a particle to pass a certain point [8] and distance=time X velocity
is the time taken by a particle to pass a certain point [8] and distance=time X velocity 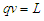
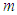 can be deduced to be
can be deduced to be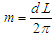
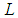 will be postulated to be a length in meter unit of the circumference of the orbital motionSo the lateral radius of the formed cone
will be postulated to be a length in meter unit of the circumference of the orbital motionSo the lateral radius of the formed cone 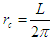
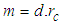
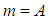 so
so 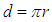
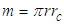
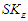 for stable particles [8] with charge
for stable particles [8] with charge 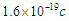 is
is 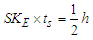
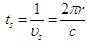
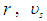 and
and 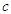 are the particle radius, frequency of spin kinetic energy, and light velocity respectively.
are the particle radius, frequency of spin kinetic energy, and light velocity respectively.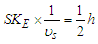
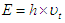 where
where 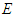 ,
, 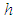 and
and 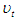 are energy, plank's constant, and frequency respectively. Multiply both sides of equation (16) by
are energy, plank's constant, and frequency respectively. Multiply both sides of equation (16) by 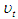 .
.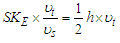
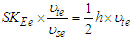
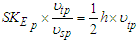
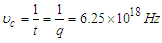 .To verify that concept for two different particles like electron and proton; equations (18, 19) have to be multiplied by the reciprocal
.To verify that concept for two different particles like electron and proton; equations (18, 19) have to be multiplied by the reciprocal 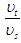 of the other particle, which will result in the orbital kinetic energy formula
of the other particle, which will result in the orbital kinetic energy formula 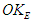 .Orbital kinetic energy for electron
.Orbital kinetic energy for electron 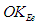
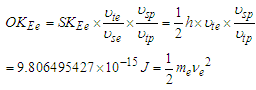
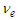 is the tangential velocity of the electron
is the tangential velocity of the electron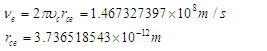
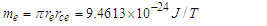
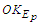
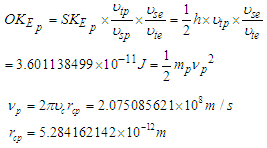
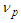 is the tangential velocity of the protonProton magnetic moment
is the tangential velocity of the protonProton magnetic moment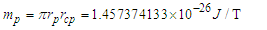
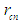 can be concluded to be a reduced radius
can be concluded to be a reduced radius 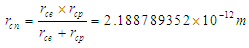 According to structure (1); neutron root mean square radius (r.m.s)
According to structure (1); neutron root mean square radius (r.m.s) 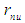
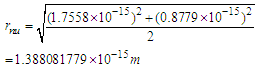 Neutron magnetic moment
Neutron magnetic moment 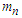
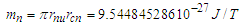
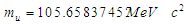 [3].And magnetic moment (-4.490 448 30 x 10-26 J T-1 ) according to CODATA [17]. Its radius can be calculated as the same as electron radius in the previous study [8] as follows;
[3].And magnetic moment (-4.490 448 30 x 10-26 J T-1 ) according to CODATA [17]. Its radius can be calculated as the same as electron radius in the previous study [8] as follows; 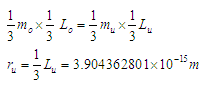
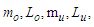 and
and 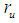 are photon mass, photon length, muon mass, muon length, and muon radius respectively.Muon orbital kinetic energy
are photon mass, photon length, muon mass, muon length, and muon radius respectively.Muon orbital kinetic energy 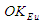
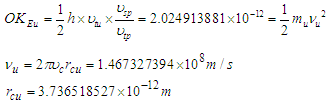
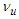 is the tangential velocity of the electronMuon magnetic moment
is the tangential velocity of the electronMuon magnetic moment 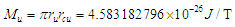
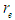 within neutron was compressed from
within neutron was compressed from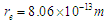 to
to 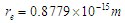 Using equations (21), (22)
Using equations (21), (22)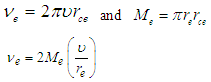
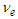 of the escaped electron in beta decay will not be constant and will depend on the ratio of the electron frequency to its radius where the electron tends to retain its original radius during escaping, as a result, the electrons will have different velocities and continuous energy spectrum.
of the escaped electron in beta decay will not be constant and will depend on the ratio of the electron frequency to its radius where the electron tends to retain its original radius during escaping, as a result, the electrons will have different velocities and continuous energy spectrum.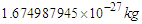 . Due to the gained energies within the neutron which are stored in the compressed electron and the connecting tail between proton and electron; the free neutron became unstable and tends to decay, its decay depends on the electron's escaping velocity and the distance which the electron has to pass for escaping.Three types of beta decay were studied and resulted in postulation that neutrino and anti-neutrino are one photon head and one positron head respectively.The magnetic moment was deduced to be the area of the cone made by the subatomic particle during its orbital motion around the orbiting center, and according to this postulation magnetic moment values for electron, proton, neutron, and muon were deduced to be
. Due to the gained energies within the neutron which are stored in the compressed electron and the connecting tail between proton and electron; the free neutron became unstable and tends to decay, its decay depends on the electron's escaping velocity and the distance which the electron has to pass for escaping.Three types of beta decay were studied and resulted in postulation that neutrino and anti-neutrino are one photon head and one positron head respectively.The magnetic moment was deduced to be the area of the cone made by the subatomic particle during its orbital motion around the orbiting center, and according to this postulation magnetic moment values for electron, proton, neutron, and muon were deduced to be 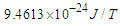 ,
,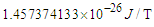 ,
,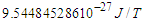 , and
, and 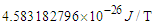 respectively. The velocity of the escaped electron in beta decay was deduced to not be a constant and depends on the ratio of the emitted electron frequency to its radius during escaping resulting in a continuous energy spectrum for the emitted beta particles.Based on this study and the previous study (photon as a basic unit); the area for a new nuclear model study will be opened.
respectively. The velocity of the escaped electron in beta decay was deduced to not be a constant and depends on the ratio of the emitted electron frequency to its radius during escaping resulting in a continuous energy spectrum for the emitted beta particles.Based on this study and the previous study (photon as a basic unit); the area for a new nuclear model study will be opened.  Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML