-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Theoretical and Mathematical Physics
p-ISSN: 2167-6844 e-ISSN: 2167-6852
2016; 6(3): 99-103
doi:10.5923/j.ijtmp.20160603.02

Evaluating Bistability in a Mathematical Model of Circadian Pacemaker Neurons
Takaaki Shirahata
Institute of Neuroscience and Kagawa School of Pharmaceutical Sciences, Tokushima Bunri University, Shido, Japan
Correspondence to: Takaaki Shirahata, Institute of Neuroscience and Kagawa School of Pharmaceutical Sciences, Tokushima Bunri University, Shido, Japan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A previous study has proposed a mathematical model of circadian pacemaker neurons in the suprachiasmatic nucleus (Sim-Forger model). The model is described by a system of nonlinear ordinary differential equations, which is based on the Hodgkin-Huxley scheme. Although it has been implied in previous studies that the Sim-Forger model shows bistability between a repetitive spiking state and a stable steady state, evidence of this bistability in this model has not been observed. Moreover, detailed evaluation of the bistability of the Sim-Forger model has not been performed in previous studies. The present study performed numerical simulation analysis of the Sim-Forger model to resolve these problems. By varying initial conditions or applying appropriate transient external stimulation, it was revealed that the model showed bistability between a repetitive spiking state and a stable steady state. In addition, it was revealed that modulation of the amplitude and timing of the stimulation was important for the stimulation-triggered transition from a repetitive spiking state to a stable steady state. These results contribute to an in-depth understanding of the characteristics of bistability of the circadian pacemaker neuron model.
Keywords: Mathematical model, Numerical simulation, Circadian pacemaker neurons, Bistability
Cite this paper: Takaaki Shirahata, Evaluating Bistability in a Mathematical Model of Circadian Pacemaker Neurons, International Journal of Theoretical and Mathematical Physics, Vol. 6 No. 3, 2016, pp. 99-103. doi: 10.5923/j.ijtmp.20160603.02.
Article Outline
1. Introduction
- Circadian pacemaker neurons in the suprachiasmatic nucleus (SCN) can spontaneously generate repetitive spiking, which is reproduced by a mathematical model based on the Hodgkin-Huxley scheme [1]. This model (Sim-Forger model) is described by a system of nonlinear ordinary differential equations (ODEs). Previous studies have evaluated several properties of the Sim-Forger model such as time courses of ionic currents during repetitive spiking [1], interspike interval changes owing to parameter variations of the model [1], and the sensitivity of dynamical states of the model to parameter variations [2]. In addition, it has previously been described in a paper that the Sim-Forger model can show bistability [3]. However, although the bistability observed in electrophysiological experiments has been shown [3], the characteristics of this bistability has not been evaluated by the Sim-Forger model in any previous study [1, 3].Bistability is one of the important characteristics in nonlinear systems. In particular, previous studies of various types of mathematical models of neurons have reported various types of bistability such as (1) bistability between an oscillatory state and a stable steady state [4, 5], (2) bistability between an oscillatory state and an oscillatory state [4-9] (the detailed classification of the type of bistability is provided in the Discussion in a previous study [10]). Experimental results from circadian pacemaker neurons [3] imply that these neurons show bistability between a repetitive spiking state and a stable steady state, which is categorized into bistability type (1). However, whether the bistability between the repetitive spiking state and the stable steady state is reproduced in the Sim-Forger model has not been confirmed in these previous studies [1, 3]. Therefore, at first, the present study investigated whether this bistability was observed in the Sim-Forger model. Previous studies have indicated the importance of a detailed evaluation of bistability by applying transient external stimulation to a mathematical model [5, 9]. However, the analysis of the circadian pacemaker neuron model by applying transient external stimulation was not performed in these previous studies [1, 3]. Therefore, in addition, in the present study, a numerical simulation of the Sim-Forger model was performed by applying transient external stimulation. The investigation of this issue will be expected to contribute to a detailed understanding of the characteristics of bistability.
2. Materials and Methods
- The present study analyzed a mathematical model of circadian pacemaker neurons (Sim-Forger model), which has been developed previously [1]. (The Sim-Forger model [1] is slightly modified in the study by Belle and coworkers [3]. However, the Sim-Forger model is an important basis of mathematical modeling of circadian pacemaker neurons, so the present study has focused on the Sim-Forger model.) The model is described by a system of six coupled nonlinear ODEs. Model equations are described as follows:
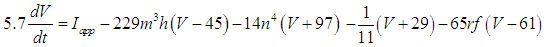 | (1) |
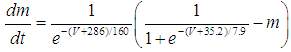 | (2) |
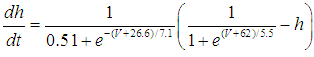 | (3) |
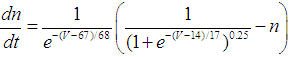 | (4) |
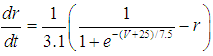 | (5) |
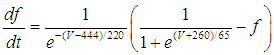 | (6) |
3. Results
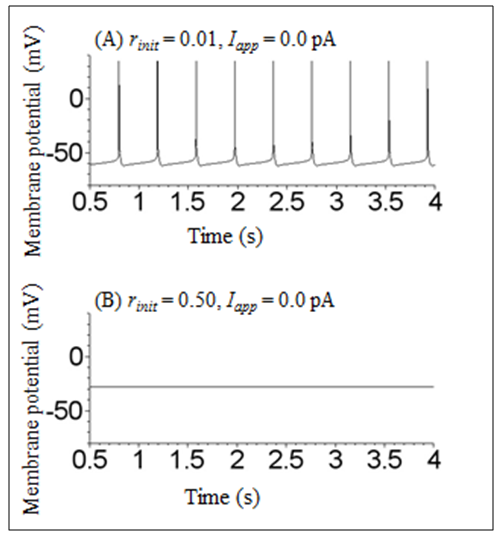
- A numerical simulation was first performed under conditions in which no stimulation was applied to reveal the type of bistability. When the initial condition was V = −80 mV, m = 0.34, h = 0.045, n = 0.54, r = 0.01, and f = 0.04, the dynamical state of the model exhibited a repetitive spiking state (Figure 1A). This spiking activity has already been described in a previous paper [1]. In contrast, when only the r value of the initial condition was increased from 0.01 to 0.50, the dynamical state of the model changed into a stable steady state (Figure 1B). These results indicate that the model shows a bistability between the repetitive spiking state and the stable steady state.
4. Discussions
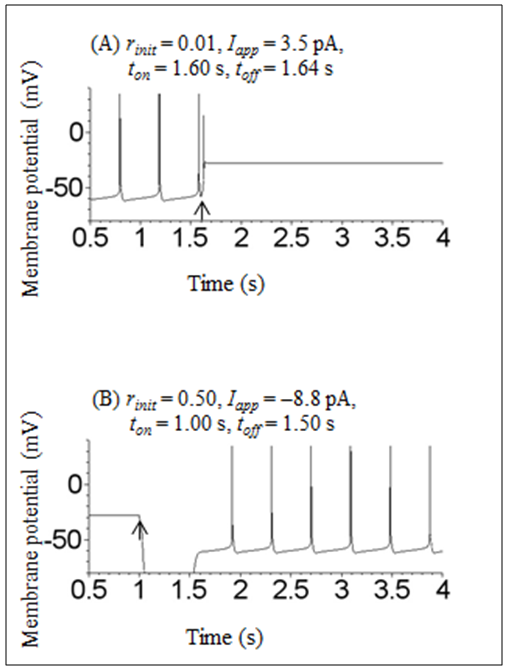
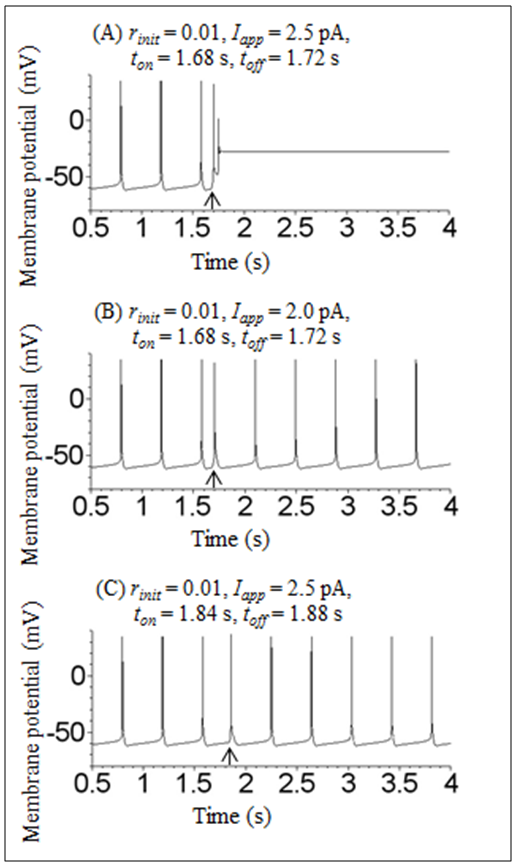
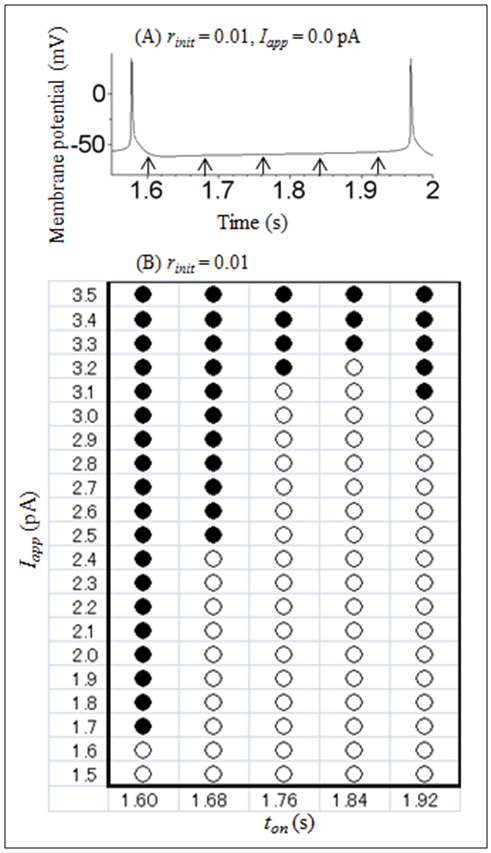
- The present study performed a numerical simulation of the Sim-Forger model, and revealed that the Sim-Forger model showed a bistability between a repetitive spiking state and a stable steady state. Transient depolarizing stimulation can change the dynamical state of the model from a repetitive spiking state to a stable steady state. In addition, the dependence of the transition from a repetitive spiking state to a stable steady state on the amplitude and timing of the stimulation was also revealed. Although bistability in circadian pacemaker neurons has already been reported in electrophysiological experiments [3], analysis of the bistability based on the Sim-Forger model had not been performed in previous studies [1, 3]. The results of the present numerical study confirmed the bistability which was implied from the previous experimental results. In addition, a novel finding that has not been reported previously [1, 3] is that it is important to regulate the amplitude and timing of the stimulation appropriately for induction of the transition from a repetitive spiking state to a stable steady state in the Sim-Forger model.The effect of transient external stimulation applied during the repetitive spiking state on the dynamics of the model is also investigated in other studies [11, 12]. However, although these studies focus on the effect of the stimulation on the cycle period of the repetitive spiking (i.e., a phase response curve), these studies did not focus on bistability. There are two other examples of studies which have investigated the relationship between the characteristics of transient stimulation and bistability. These include an analysis of a mathematical model of leech interneurons [5] and snail pacemaker neurons [9]. However, it was found that there were two differences between the present and previous studies. The first is the type of bistability. The study of the leech interneuron model investigates the transition from a periodic bursting state to a stable steady state [5], while the study of the snail pacemaker neuron model investigates the transition from a periodic bursting state to a chaotic spiking state [9]. In contrast, the present study investigated the transition from a repetitive spiking state to a stable steady state. The second is the dependence of the transition of the dynamical state on the stimulation amplitude and timing. The study of the leech interneuron model reports that the depolarizing stimulation is effective for transition at the early timing of a silent phase of bursting, while the hyperpolarizing stimulation is effective for transition at the late timing of a silent phase of bursting [5]. The study of the snail pacemaker neuron model reports that the hyperpolarizing stimulation is effective for transition at both early and late timings of a silent phase of bursting [9]. The present study indicated that the depolarizing stimulation was effective for transition at early, intermediate, and late timings of a silent phase of repetitive spiking in the Sim-Forger model (Figure 4B).
5. Conclusions
- The present numerical study of the Sim-Forger model revealed that the model showed bistability between a repetitive spiking state and a stable steady state, and appropriate transient depolarizing stimulation was able to induce a transition from a repetitive spiking state to a stable steady state. In addition, it was revealed that it was important to regulate the amplitude and timing of the stimulation for induction of the transition, which was not reported in previous studies of the Sim-Forger model. Compared with the previous studies, the present study can contribute to a more detailed understanding of the characteristics of the bistability of circadian pacemaker neurons.
ACKNOWLEDGEMENTS
- The author would like to thank Enago (www.enago.jp) for the English language review.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML