-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Stomatological Research
2012; 1(3): 24-30
doi: 10.5923/j.ijsr.20120103.02
Clinical Effects of Systemic Azithromycin as an Adjunct to Scaling and Root Planing in the Treatment of Chronic Periodontitis: A Randomized Placebo-Controlled Clinical Trial
Seyyed Ali Banihashemrad 1, Majid Reza Mokhtari 1, Mohammad Hassan Najafi Neshli 1, Golsa Akbarian 1, Fatemeh Farazi 2
1Dept of Periodontology,School of Dentistry,Mashhad University of Medical Sciences,Mashhad,91889,Iran
2Dept of Oral Medicine,School of Dentistry,Bojnourd University of Medical Sciences,Bojnourd,49815,Iran
Correspondence to: Majid Reza Mokhtari , Dept of Periodontology,School of Dentistry,Mashhad University of Medical Sciences,Mashhad,91889,Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Antibiotics are used adjunctively to scaling and root planing (SRP) for treatment of periodontal disease. The aim of this study was to evaluate the clinical effects of systemic Azithromycin (AZM) as an adjunct to SRP in the treatment of patients with chronic periodontitis. This study was a double-blinded, randomized clinical trial in which 49 patients with chronic periodontitis participated. Patients were randomly divided into 2 groups: The test group which received SRP plus AZM and the control group which received SRP plus placebo with the same dose. Clinical indices including Probing Pocket Depth (PPD), clinical Loss of Attachment (LOA), Gingival Index (GI),and Plaque Index (PI) were measured at the baseline and 3, 13, and 25 weeks after treatment.The mean LOA and PPD in the test group were significantly less than the control group after 25 weeks from baseline. Over all, the mean LOA and PPD were less in the test group than the control group at all times. All four mentioned indices showed further reduction in the test group during time.The adjunctive use of systemic azithromycin show significant clinical benefit in the treatment of chronic periodontitis.
Keywords: Periodontitis, Treatment, Azithromycin
Cite this paper: Seyyed Ali Banihashemrad , Majid Reza Mokhtari , Mohammad Hassan Najafi Neshli , Golsa Akbarian , Fatemeh Farazi , "Clinical Effects of Systemic Azithromycin as an Adjunct to Scaling and Root Planing in the Treatment of Chronic Periodontitis: A Randomized Placebo-Controlled Clinical Trial", International Journal of Stomatological Research, Vol. 1 No. 3, 2012, pp. 24-30. doi: 10.5923/j.ijsr.20120103.02.
Article Outline
1. Introduction
- Chronic periodontitis is believed as an infectious disease with a bacterial etiology causing inflammation and progressive devastation of the tooth supporting tissues (1, 2). Pocket formation, loss of gingival attachment and alveolar bone resorption are some of its evidences. The conventional therapy for periodontal diseases consists of mechanical debridement of teeth surfaces (scaling and root planing) or surgical treatment in order to make a better access for root instrumentation (3, 4). However, it has been suggested that administration of antibiotics adjunctively to SRP can present better clinical and microbial results in comparison to SRP alone (5,6,7,8). Azithromycin is a macrolide antibiotic in related to erythromycin with a considerable potency against gram negative organisms and a few side effects (9,10,11). It is quickly absorbed by neutrophils, macrophages and fibroblasts which make a fast delivery of the drug in infected tissues. It is believed that concentration of AZM is 10-100 times higher in tissues than in serum. Besides, it has a long half-life related to its prolonged deliverance to the tissues which makes it to be recommended for a short period of time (12, 13). Several investigations have reported the clinical results of utilizing AZM. Mascarenhas P et al (14) have reported the effectiveness of AZM in reducing probing depth and improving attachment levels compared to SRP alone in smokers with periodontitis. Hirsch et al (15) have also demonstrated the beneficial effects of AZM in the treatment of periodontal diseases. On the other hand, Angaji M et al (16) showed that there was inadequate and indecisive evidence for supplementary efficacy of adjunctive antibiotic therapy on chronic periodontitis in smokers according to their systematic review article.The aim of this study was to evaluate the clinical effects of systemic AZM as an adjunct to SRP in the treatment of patients with chronic periodontitis in order to achieve a beneficial therapeutic diet.
2. Methods and Materials
2.1. Trial Design, Participants and Sample Size
- This study was a double-blinded, randomized and placebo-controlled clinical trial. Eligible patients were selected from those with chronic periodontitis who had been treated in the Department of Periodontics at Mashhad Dental School (Mashhad, Iran) during 2008-2009. 83 patients with chronic periodontitis were assessed and oral examination and medical history review were gathered to confirm eligibility. 33 patients left the study since they did not meet inclusion criteria. Eventually, 50 subjects signed a written informed consent and allocated the study. One of the patients, in the control group, lost to follow-up in the third period of the study because of using tetracycline. The inclusion criteria were: medically healthy patients with untreated moderate to severe chronic periodontitis, having more than 12 teeth (excluding third molars and teeth with orthodontic appliance, bridges, crowns, and implants), having at least 4 posterior teeth with PPD>4mm and clinical attachment loss (AL)>2mm, and presenting radiographic evidence of alveolar bone loss.Patients were randomly divided into 2 therapeutic groups using a computer-generated random numbers table: The test group (25 patients) which received SRP plus AZM (one pill (250mg) the day before scaling, 2 pills for first day, and one pill a day for four days after scaling), and the control group (24 patients) which received SRP plus placebo with the same prescription. This trial was approved by the Ethics Committee of Mashhad University of Medical Sciences (Mashhad, Iran registry code#87757). Based on Sefton AM et al (24) and Smith SR et al (25) studies, with β=0.2 , α =0.05 sample size was measured as 25 patients in each group.
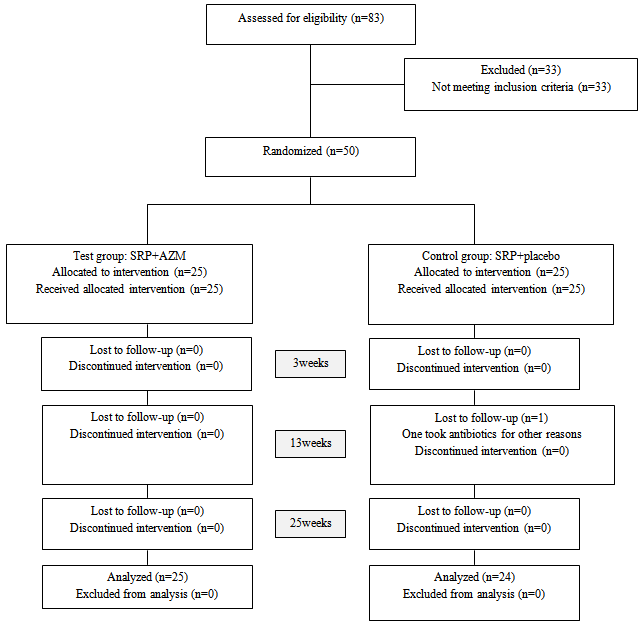 | Figure 1. flow diagram of the study design |
2.2. Assessment of Subjects
- Clinical indices including PPD (Probing Pocket Depth), LOA (Loss of Attachment), GI (Gingival Index), and PI (Plaque Index) were measured at baseline (right before the treatment) and 3, 13 and 25 weeks after the treatment. PPD was recorded from the gingival margin on the mesial, buccal, distal and lingual aspects of teeth with a William's periodontal probe (Hu Friedy, Chicago, IL, USA). LOA was measured from the cemento-enamel junction to the base of the pocket on the mesial, buccal, distal and lingual aspects of teeth. GI was determined according to established GI criteria (17). PI was measured according to Silness and Loe (18) and patients were informed about the important role of dental plaque in periodontal diseases.
2.3. Periodontal Treatment
- Before the first treatment visit, each subject was given a code number and the examiner recorded all patients' medical history and prescribed the medicines. Both groups of medicine (AZM or placebo) were presented in apparently identical package by one of the researchers who marked the code number of each subject on each package according to the therapy assigned. The coded package was given to the examiner who was blind throughout the study. Moreover, the operator and all patients were also blinded.At the first visit, right before the SRP treatment, the examiner recorded all clinical indices and removed the plaques above the gums and trained the accurate method of mouth cleansing. Then the operator performed the scaling and root planing and polishing of the involved teeth under local anesthesia. Ultrasonic devices (VGE 302k, Juya Electric Co., Tehran, Iran) and Gracey curettes were carried out respectively. All patients were recalled 3, 13 and 25 weeks after the baseline treatment. The mentioned four clinical indices including GI, PI, LOA, and PPD were measured and recorded in specific tables during each follow-up visit. Mouth sanitary was checked and amplified and no re-instrumentation of the leftover periodontal pockets was done throughout the study.
2.4. Statistical Analysis
- The statistical tests included: Kolmogorov-Smirnov, t-test, Chi-Square, Fisher exact test, Repeated Measures and Bonferoni test. After using Kolmogorov-Smirnov test it has been demonstrated that data were normally distributed. Repeated Measures and Bonferoni test were performed to detect significant differences within each group throughout the course of trial. T-test was used for comparison of each clinical parameter between 2 treatment groups in each time point. SPSS software was carried out for data analysis. The probabilities of less than 0.05 were considered as statistically significant.
3. Results
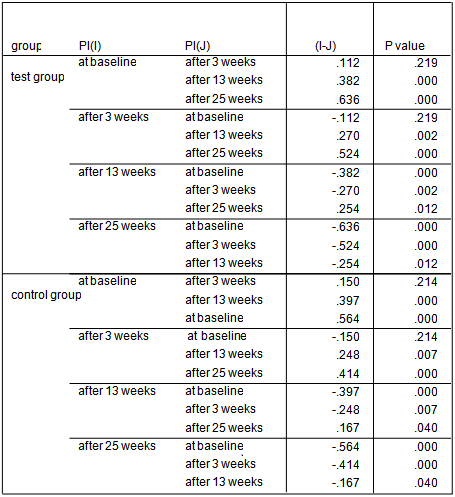
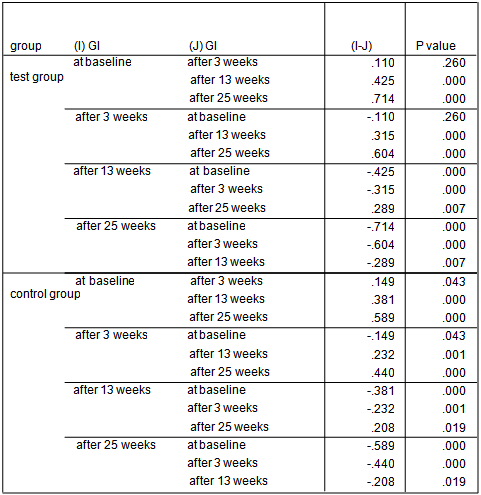
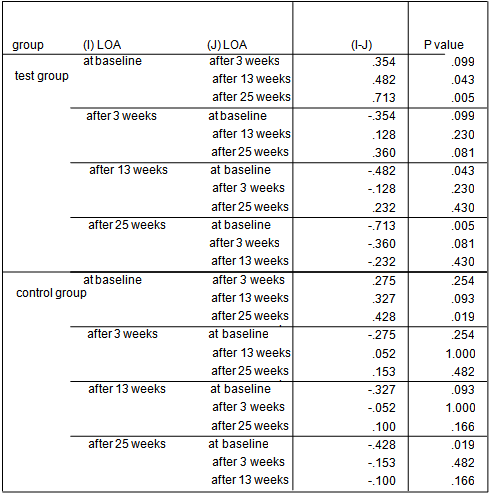
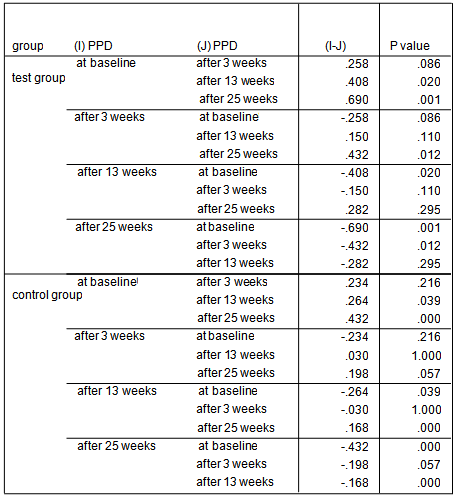
- The average age of patients in the control group was 34.83±1.42 years, and in the test group was 32.4±1.5 years (P=0.24). Women constituted 68% of patients in the test group and 50% in the control group (P=0.20).16.3% of the participants did not brush and 59.2% brushed only once a day. 81.6% didn’t use dental floss however, 18.4% used it for at least once a day. 54.2% of individuals in the control group and 52% of them in the test group experienced bleeding from their gums during brushing (P=0.87). Comparing each clinical index (PI, GI, LOA, PPD) between two groups at each single time point showed significant reduction of LOA (p=0.004) and PPD (p=0.00), after 25 weeks in the test group compared to the control group. There was no significant difference of other indices between the two groups at each time.
|
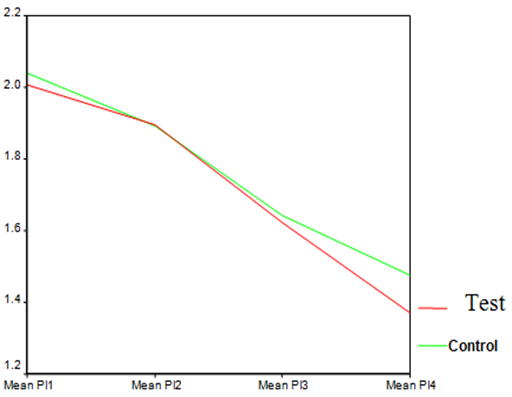 | Graph 1. PI in test and control groups at different time points |
|
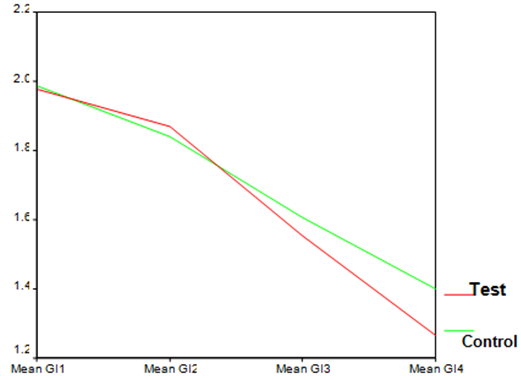 | Graph 2. GI index in test and control groups at different time points |
|
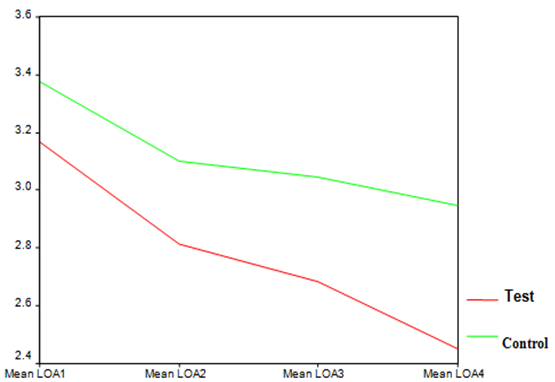 | Graph 3. LOA index in test and control groups at different time points |
|
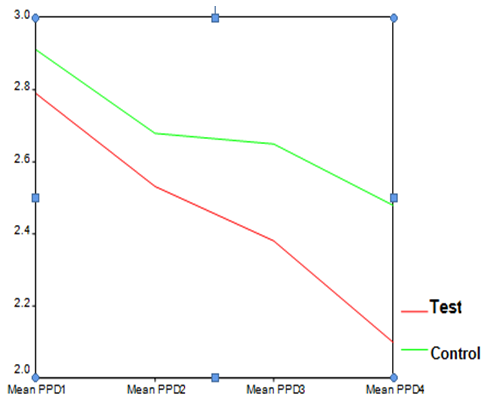 | Graph 4. PPD index in test and control groups at different time points |
4. Discussion
- The present study evaluated the clinical effects of AZM as an adjunct to SRP in the treatment of patients with chronic periodontitis. Mechanical debridement leads to disrupt the dental biofilm. Therefore, using systemic antibiotics reduces the bacterial load and minimizes the inflammation in the periodontal pocket (19). A wide range of antibiotics have been used as an adjunct to non-surgical treatment of periodontitis (20,21,22). Azithromycin is one of these antibiotics with special pharmacokinetics properties and few side effects which is simply accepted by patients due to its short course of administration and its uncomplicated regimen (23,24). All our patients took their medicines thoroughly according to the prescription and none of them showed any relevant adverse reaction. Our study revealed that patients who had received Azithromycin showed significant reductions of the mean LOA and PPD after 25 weeks in comparison to the subjects who had only received SRP. This is in accord with the study by Sefton AM et al (24) which has reported that the mean PPD of pockets which were more than 6 mm deep at baseline was significantly lower in the azithromycin treated group after 22 weeks. However, the PPD reduction of pockets with initial depths of less than 3mm was the same in both groups at that time. This is related to the fact that the remarkable role of AZM is mostly observed in deep periodontal pockets and those with moderate to severe periodontitis(25). They have also concluded that there may be beneficial microbiological and clinical affects of AZM as an adjunct to SRP at least in a short period of time. Smith SR et al (25) have also demonstrated that pocket depths initially 4-5 mm or 6-9 mm presented significantly lower mean pocket depth in subjects who had been treated with AZM (p<0.01). On the other hand, Sampaio E et al (12) have stated that both therapeutic groups (AZM+SRP and SRP alone) presented identical improvement of the mean CAL (Clinical Attachment Level) and there was no significant difference between them at any time point.Overall, the clinical results of both groups in the present study, improved over time, although patients who had taken AZM presented further clinical development compared to those who had only received SRP. The only exception is related to the mean PI and GI after 3 weeks which were less in those who had only received SRP (P>0.05). This is consistent with the study by Smith SR et al (25) that reported all patients irrespective of treatment group (AZM+SRP or SRP alone) showed reduction of PI without significant difference at any time point. Hirsch R (23) and Schmidt EF et al (26) have reported reduction of PPD, BOP (Bleeding On Probing) and gingival inflammation, also regeneration and consolidation of alveolar bone observed in their cases with chronic periodontitis. Hirsch R (23) have surprisingly noticed alveolar bone regeneration and clinical improvement in one of the patients who had only received AZM with no periodontal intervention, although it is recommended not to consider antibiotics as a monotherapy in the treatment of periodontitis (19). Haffajee et al (27) have evaluated clinical results of four different periodontal therapies (AZM, metronidazole and doxycycline adjunct to SRP compared to SRP alone) in the treatment of chronic periodontitis. Their analysis revealed that all groups which had received adjunctive antibiotics showed further clinical improvement (reduction of PPD and LOA and sites with suppuration) in comparison to the group which had only received SRP. However, the difference was not significant. In addition, the mean PPD and LOA of the pockets with more than 6mm depth were significantly less in the subjects treated with AZM and metronidazole compared to other groups. This may be because of unique pharmacokinetics properties of AZM (such as its high concentration and prolonged deliverance to the infected tissues) and its short period of administration which makes it easer for patients to take properly.
5. Conclusions
- According to the present study, the adjunctive use of systemic azithromycin show a significant clinical benefit in the treatment of chronic periodontitis in comparison to SRP alone and it is suggested for additional studies by other antibiotics and specially combination antibiotics as adjunct to non-surgical periodontal treatments .
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML