-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Stomatological Research
2012; 1(2): 6-16
doi: 10.5923/j.ijsr.20120102.01
Periodontium and Orthodontic Implications: Biological Basics
Marinho Del Santo
Orthodontist in Private Practice, Neo Face Dental Clinic
Correspondence to: Marinho Del Santo , Orthodontist in Private Practice, Neo Face Dental Clinic.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
As more and better orthodontic and periodontal scientific evidences are contextualized, potentially richer will be their achieved clinical results. The primary goal of this article is to study the main anatomic, histologic, physiologic and pathologic features of the periodontal tissues, and such knowledge to be applied in the Orthodontics and Periodontics fields.
Keywords: Periodontics, Orthodontics, Periodontal Development, Periodontal Anatomy, Periodontal Physiology
Article Outline
1. Introduction
- In general, the periodontal tissues of patients who seek periodontal treatment suffer constant and dynamic oscillation between the physiologic and pathologic states, and the natural ongoing process to achieve periodontal homeostasis depends upon tissue reposition, remodeling, degradation and repair. In practice, as more and better orthodontic and periodontal treatments are contextualized, more potentially richer will be the achieved clinical results.In Orthodontics, dental movements target the correction of malocclusions. Such goal includes the leveling and alignment of the teeth in the arches, and such movements occur throughout dynamic processes of bone resorption and bone deposition. The correct dental positioning allows a better occlusal load distribution and facilitates the routinely oral hygiene of the patient. Therefore, orthodontic results increase the masticatory action, eliminate occlusal interferences and increase the probability of maintenance of periodontal health of the treated patient. Since Orthodontics allows that the protective periodontium (gingival tissues) fits better around better positioned teeth, it facilitates hygiene care and contributes for the esthetical aspects of smiling. However, when biological limits are not respected by the applied orthodontic mechanics, partial relapse of the achieved results and alveolar and root resorption may occur.In Periodontics, the clinical or surgical therapy promotes a healthy periodontal status and targets to carry on such condition. However, the physiological periodontal homeostasis may not be maintained because diverse temporal and individual factors as oral hygiene, microorganisms virulence, integrity of defence barriers ofthe tissues and host resistance. When the equilibrium is broken the periodontal disease is installed or re-installed, and diverse degenerative levels can possibly happen, and are revertible or not.
2. Goal
- The main goal of this article is to study the periodontal tissues, illustrating their innate features, and highlighting pertinent clinical parameters. The target-specialists of these publications practice in the Periodontics and Orthodontics fields, and must know the mandatory periodontal requisites to recommend orthodontic treatment, and the benefits of the orthodontic treatment for the periodontal homeostasis.
3. Panoramic View: Periodontal Protection and Function
- Independently of clinical and experimental advances, the most basic and undeniable biological principle in Periodontics is: the periodontium is made by protective and supportive tissues which allow the teeth to be present in the oral cavity and perform its main function mastication, indeed the discontinuity of the oral mucosa do not make it vulnerable to alien antigens.The human body (and also of other mammals) is protected against alien microorganisms by the continuity of its tissues, fundamentally the skin and the nasopharyngeal and gastrointestinal mucosa. With dental eruption, the continuity of the oral mucosa is broken and in order to maintain the protection of the individual, a sophisticated system of biological sealing grows and develops around the teeth. Such system is the protective periodontium, mainly the junctionalepithelium. However, other factors also play important role for the scenario where periodontal homeostasis is achieved and maintained, as the correct and complete oral hygiene performed by the patient.Discipline and motor-skill are fundamental for the patient to achieve and maintain such goal, mainly targeting the equilibrium between the periodontal microbiota and its host, “protecting the protection”. The junctionalepithelium must be protected of suffering more intense aggressions that is naturally prepared to do so. Professionals deliver such equilibrium status to the patient and orient him/her how to maintain a satisfactory level of microorganism colonization. In our opinion, to discriminate periodontal tissues as protective or supportive,110 although histologically correct, is unnecessary. The periodontium, under a broader view, is a unique and highly sophisticated system. The gingiva is prepared to receive the masticatory load and establish a protector collar, fibrous and well attached, around the teeth. Its junctionalepithelium represents the biological sealing of the “internal periodontium environment” against external aliens. Its periodontal ligament promotes the dynamic mechanical-functional relationship between root Cementum and its respective laminate alveolar bone. Therefore, the periodontal structures shall be studied and understood as specialized epithelial and connective tissues that, up to certain limits, are philogenetically adapted and epigenetically lapidated according to the imposed functional demand.
4. Embryological Origin and Development of the Periodontium
- As a rule, the periodontium is a tissue of ectomesenchimal origin. However, its gingivalepithelium (including the junctionalepithelium) and the rest of Malassez cells have origin exclusively ectodermic. The gingivalepithelium plays important ontogenetic modulation in the ectomesenchimal tissues,47,111,112,114 although such inductive mechanisms are not enough clear.128 The enamel organ forms enamel, the dental papilla forms the dental-pulp complex and the dental follicle forms the Cementum, the periodontal ligament and partially the alveolar bone.As examples of the high complexity specialization of the periodontal tissues, we highlight the junctionalepithelium and the periodontal ligament. The junctionalepithelium represents a protective tool. Without it there would be no periodontal sealing and the external and internal environments were not isolated one from each other. The periodontal ligament is a complex apparatus for mechanic-functional support of the teeth, allowing their relationship with the alveolar bone.
5. Anatomy-Histology-Physiology of the Periodontium
- The oral mucosa is continuous with the labial vermillion and with the soft palatal and pharyngeal mucosa. It is divided in: 1) masticatory mucosa, keratinized, which represents 25% of the internal oral surface, it is similar to the skinepithelium and it is present in the gingiva and the hard palate; 2) specialized mucosa, which covers the tongue dorsum and; 3) oral mucosa, non-keratinized, which represents 60% of the internal oral surface, it is similar to the esophagical and vaginal mucosa, it is continuous to the masticatory mucosa and it is present in the rest of the mouth.As part of the masticatory mucosa, the gingiva is a fibrous collar tissue around the teeth, resistant, continuous and well attached, inserted in the teeth and in the crest alveolar bone. The gingiva overlaps approximately 2.0 mm the enamel-Cementum junction, being that its sulcus measures approximately 0.5 mm 127 and the junctionalepithelium measures approximately 1.5 mm. With the installation of the gingivitis process, the deepness of the gingival sulcus may increase, not because of some apically migration of the junctionalepithelium, but because inflammation swallows the gingiva. With the possible progression of gingivitis the junctionalepithelium breakage occurs and, consequently, a direct communication between the external and internal environments. An evident sign of such continuity is gingival bleeding.epithelium per se can not bleed since has no blood supply.On the teeth, bellow the junctionalepithelium, there is approximately 1.0 mm of fibrous gingival attachment: dental-gingival and dental-periodontal fibers. Therefore, the total space between the gingival free margin and the crest of the alveolar bone is approximately 3.0 mm. It is called biological space. The gingival collar is also formed by alveolar-gingival, circumferential and semi-circumferential, trans-gingival, inter-gingival and trans-septa fibers, composing a resistant soft tissue frame which contours all the teeth. Such fibrous bundles are mainly made by type I collagen (approximately 91% of incidence; it is the dense collagen) and type III collagen (approximately 8% of incidence; it is the soft collagen).69,70,22 Such net of collagen fibers, with longer turnover than skin fibers,99 besides providing consistency and resistance to the gingiva, promotes its attachment to teeth and to the alveolar bone, making a supportive apparatus for the masticatory function. Moreover, such twisted fibrous system maintains the adjacent teeth inter-connected in the dental arch. The referral of pre-orthodontics dento-gingival fibrotomy targets to minimize the resistance to the orthodontic movement and also to decrease the risk of relapse, in special when dental rotation is the case.50Anatomically, the gingival tissue is adapted to the shape, size and positioning of the teeth. The gingival shape is of continuous segmented arches separated by inter-dental papillae. In the inter-proximal regions the gingiva is narrow between the anterior teeth and large between premolars and molars. In young patients, the inter-proximal gingiva totally fills the space between the teeth, in a region called gingival col. Attached gingiva is the band of gingival tissue present between the free gingiva and the mucosa-gingival junction. The attached gingiva is fixed, pink (or pigmented in afro-descendent individuals), more or less thick and more or less large. Its thickness ranges approximately from 0.5 mm to 2.5 mm and its width ranges approximately from between 3.0 to 6.0 mm, being thickest in the vestibular face of the upper incisors and in the lingual face of the lower incisors.32 The thickness of the attached gingiva also varies according to the degree of dental eruption.63,64,88 In the other hand, the oral mucosa is mobile, thin and relatively elastic, being highly blood supplied (with reddish appearance).The oralepithelium is a stratified pavedkeratinizedepithelium, presenting four cellular layers: basal, spinosum, granulosum and keratinized (differentiated or not). The cells of the granulosum and keratinized layers exfoliate on the tissue surface with a turnover of approximately 14 days. The keratinocytes are cells that produce keratin (and make the keratinized layer) and correspond to 90% of the total cellular tissue population. The keratinized layer is the principal barrier against external aggressions. Besides keratinocytes, melanocytes, Langerhans cells, Merkel cells and inflammatory cells are also found. The oralepithelium does not present inter-cellular fibrous components but proteoglycans,121,7 adipose tissue and water. The proteins lectin, hyaluronan, chondroitin, dermatan, decorin and sindecan,79,98,103,52,82 among others, are in tight contact with the adjacent connective tissue.75 The gingivalepithelium is made by the oralepithelium, sulcusepithelium and junctionalepithelium.
6. Junctionalepithelium
- With dental eruption, the fusion of the enamel reduced organepithelium with the oralepithelium occurs. The dental-gingival junction is established as anepithelium collar, derivated from the two epithelia, which united to make the junctionalepithelium.93 The junctionalepithelium and the sulcularepithelium recover the gingival sulcus, where protection is the main goal. The junctionalepithelium is a simpleepithelium, constituted by only two layers (strata) of stratified squamousepithelium, with cuboid basal cells and flat supra-basal cells, parallel oriented to the dental surface. It is not keratinized and presents few desmosomes for inter-cellular union,90,35 with inter-cellular spaces that shelter a great quantity of neutrophils, macrophages, mastocytes and lymphocytes, present with or without inflammation.91 The sulcular fluid, secreted even without any detected pathological process,20 contributes to the mechanical cleanness of the gingival sulcus and it may be accumulated in its bottom.The junctionalepithelium presents an exceptionally high turnover, being completely substituted in approximately 05 days in humans.19 Such replacement is promoted by constant mitosis in its basal layer and consequent exfoliation of supra-basal cells into the gingival sulcus. The basal layer of the junctionalepithelium can regenerate from the basal cells of the oralepithelium and also, potentially, regenerates from the skin.55,19 Such regeneration is also possible around dental implants.33,65,34 As the junctionalepithelium presents a significant amount of polymorphic leucocytes, it might be compared to theepithelium of lymphoid tonsils92,94,80 and it is considered a very favorable environment for immunological reactions, innate or acquired.80 If the inflammatory process is installed, polymorphic leucocytes are attracted by microbiological chemotaxis and abundantly released in the gingival sulcus.105,2 As important the inflammatory process is, as greater neuthophil migration occurs,3,48 providing the first innate immunological combat against external aggressors. Macrophages and lymphocytes are also found; however, they are less than 5% of the polymorphic leucocytes.2 Lymphocytes leads acquired immunological reactions.Clinically, adequate oral hygiene allows reduction or elimination of bacterial aggressive agents in the gingival sulcus. In an adequate ecosystem, possible repair and reinstallation of a junctionalepithelium in a more coronal position, in natural teeth and in dental implants, is the best periodontal protection. Theepithelium is called of long junctionalepithelium. Even assuming that the losses in the alveolar bone crests are irreversible, the possibility of establishment of a long junctionalepithelium significantly improves the prognosis of an adverse periodontal scenario.
7. Dentin-Cementum Junction
- Genetic expression for the development of the dental organ occurs in the dental papilla and in the dental follicle.112 The Hertwig´s epithelial root sheath is classically described as the precursor for the apical development of the dental organ, in its dentinoblastic and cementoblastic differentiation.116,115,60,61 The hyaline layer of Hopewell-Smith, originally described by Hopewell-Smith,38 can be a variation of the dentin which recovers the dental root surfaces. It was described it as non-cellular Cementum.49,77 It has been confirmed73,74,14-18 the possible existence of such non-mineralized layer between the dentin the Hertwig root sheath, continuous with the dentin and therefore called pre-dentin; however, its existence is only sporadic.109 In theory, the function of the Hopewell-Smith layer would be to promote non-cellular Cementum deposition over root dentin.112
8. Cementum
- Cementum is a mineralized connective tissue, specialized in the recovering of the dental roots and where the collagen fibers of the periodontal ligament are anchored to promote dental sustain.77 Cementum presents peculiar biological features.The cementoblasts, cells that produce Cementum, are originated in the dental follicle.106,114 The interactive relationship between dentinoprogenitor cells and Cementumprogenitor cells16 indicated that there is no gap between dentin and Cementum in humans.17 However, the origin of Cementumprogenitor cells is controversial. Although in theory they are originated from the ectomesenchyme, the hypothesis of that they are originated in the Hertwig´s sheath has not been refused.116,61 There are different structural types of Cementum, according to its location, function, chemical composition and mineralization degree.Basically, human teeth present three types of Cementum: 1) the non-cellular and non-fibrous type, which recovers small areas of the enamel, near to the enamel-Cementum junction; 2) the non-cellular fibrous type, which is present in the two coronal thirds of the dental root and; 3) the cellular fibrous type, which is present in the apical third of the dental root. However, in the anterior teeth the non-cellular fibrous type also covers part of the apical third of the root, and such coverage decreases in the posterior teeth, where more cellular fibrous type Cementum is identified. Cellular Cementum is present where the non-cellular Cementum is not present, as furcations and apices. In general, Cementum is thicker as apical as it is located, and it can invade the apical foramen.The non-cellular Cementum is more mineralized than the cellular Cementum, possibly because its process of formation is slower. Then, it allows longer contact of the minerals, that come from blood vessels, with the organic matrix. The apical cellular Cementum holds the capacity of quicker growth when compared to the non-cellular Cementum. It also presents more intense remodeling, in response to the masticatory functional stimuli from dental occlusion. It seems that such functional stimuli reduce the mineral deposition rate in the apical area and do not increase it. The cellular Cementum can overlap layers of non-cellular Cementum and vice-versa.The composition of the Cementum matrix is similar to the bone matrix and to the dentin matrix, being 50% of it made of inorganic components, mainly hydroxypatite crystals. Apparently, the Cementum mineralization process is identical to the bone mineralization. Its framework is made by a collagen matrix, where mineral deposition occurs. Such organic matrix is mainly made by type I (90%) and type III collagen (5%) 11,18,122 and by associated adhesion proteins as condroitin,4,6 sialoprotein, tenascin and osteonectin.61 It has been suggested that type I fibers are recovered by type III fibers,120 avoiding its mineralization. However, other authors hold different opinion, stating that type I and type III collagen are parallel to each other, and there is no overlap.42,9,40 Interesting to note that although the new layers of Cementum are added during the lifetime of the individual, its mineral contend does not significantly change with aging,95,68 differently of what happens with dentin, which mineral contend significantly does, provoking obliteration of the dentinary channels.Bone participates of the systemic metabolism, as a reserve of Calcium and other minerals; however, Cementum is excluded of such dynamics. There is no evidence that changes in the Calcium systemic level are associated with biochemical changes in the Cementum. It is possible that Fluoride ions brought by blood vessels of the periodontal ligament plays a role. It intensively reacts with the hydroxyapatite of the Cementum surface, especially in the dental cervical region, and it armors Cementum against Calcium systemic biochemical changes. Although do not suffer continuous remodeling, Cementum slightly increases its thickness during lifetime, if no periodontal disease is present. Intense Fluoride deposition on Cementum surface is probably the main reason for the fact that caries and root resorption are presented in a “mined form”, that means, they progress internally, before significant major changes on the Cementum surface.Usually Cementum is classified of a “bone-like” tissue; however, the comparison is limited. The differences between both tissues are evident when bone and Cementum biodynamics are studied during the orthodontic movement. Quantitatively, Cementum responds to tension orthodontic forces as alveolar bone; however, in a much slower speed. In the side submitted to orthodontic tension, slight Cementum deposition may occur in layers.81 Under physiologic orthodontic conditions, in the side submitted to orthodontic pressure, alveolar bone suffers resorption and Cementum does not, or if does, it is not evident by routine diagnostic methods. This difference between both tissues is essentially the speed of the metabolism: the bone metabolism is much faster and dynamical than the Cementum metabolism.And there are histological reasons for such difference. The main reason is blood supply, absent in non-cellular Cementum and minimal in cellular Cementum; however, abundant in alveolar bone. As a consequence of such minor blood support, with sparse and limited channel communications, the Cementum turnover is much longer that the bone one. Besides that, although the collagen fibers of Cementum and alveolar bone are both exposed to the intense metabolic activities of the periodontal ligament, the fibers that are inserted in the Cementum present a turnover significantly longer than the ones of the alveolar bone. In the Cementum, the attached fibers are more numerous, more stable and more mature. In the alveolar bone, the fibers (Sharpey´s fibers) are unstable and suffer relatively faster remodeling.54In adverse orthodontic conditions, as excessive orthodontic load combined or not with extensive orthodontic treatment, Cementum resorption may also occur. In such conditions, bone resorption is also altered: it is delayed and it occurs “internally” and not “superficially”, due to the obliteration of the blood vessels of the periodontal ligament, with consequent necrosis and tissue hyalinization. As a direct clinical consequence, easily assessed, the orthodontic movement is delayed. Up to the timing that necrosis and tissue hyalinization are solved by the installed inflammatory process, possible Cementum resorption may happen and may also be repaired. Most of the time, it is not even diagnosed. When root resorption is evident, showing shortening of the root length and rounding of the dental apice, the process is already in an advanced phase.Under adverse conditions, dental ankylosis may also occur. Dental traumas provoke ankylosis as a consequence of the tissue damage and bleeding in some sites of the periodontal ligament and the alveolar bone. The tissue reparative response promotes undesired fusion between Cementum and alveolar bone. However, ankylosis may also have other reason: lack of masticatory function. In this scenario, lack of demand of tissue remodeling leads to atrophy of periodontal ligament fibers. The bone-Cementum fusion can be an important consequence of such atrophic state.
9. Periodontal Ligament
- In the human specie (and in other mammals) teeth are not rigidly united to the alveolar bone, but articulated to it by a fibrous connective mesh, called periodontal ligament. The periodontal ligament is a soft connective tissue, multifunctional and with unique proprieties, not showed by other tissues.10 The periodontal ligament is specialized in support, protection and sensorial proprioception of the masticatory system. It is a mesh of collagen fibers, of approximately only 0.2 to 0.3 mm of thickness, organized according to its functional purpose. Moreover, the periodontal ligament is the source of the progenitor cells of the anatomic-functional unit called supportive periodontium.Although the fibroblasts of the periodontal ligament can not be originated from gingival cellular populations,51 both tissues have their development closely related. The gingival connective tissue and the periodontal ligament are fused during the dental eruption process and there is no anatomic distinction between both.76 Besides the fibroblasts, the periodontal ligament presents endothelial cells, rest of Malassez epithelial cells, nervous sensorial system cells, blood vessels, cells associated with bone formation (formation of the alveolar bone radiographically called lamina dura) and cementoblasts.10 As other gingival tissues, most of the fibers of the periodontal ligament are made of type I and type III collagen,21,120,70,71,22,40 that are firmly attached to Cementum.108 The type V and type XII fibers are present in smaller amount. Indeed, the blood vessels of the periodontal ligament present types I, III, IV and V collagen fibers.8 The extra-cellular matrix that holds periodontal ligament fibers was studied in details in sheep.45 In such matrix there is small amount of elastin and tenascin,23,5,59 but the same proteoglycans of the gingival connective tissue were found. As examples, hyaluronan, heparan, dermatan and chondroitin,78,31 and versican and decorin,78 are intrinsically imbricated among collagen fibers, cells and vessels. The biochemical composition of the proteoglycans of the periodontal ligament is altered in consequence of periodontal disease46,44 and during orthodontic movement.83,28,53,100One of the main functional features of periodontal ligament is its extremely high capacity of remodeling under local changes, presenting short cellular and protein turnover. The turnover of the collagen fibers of the periodontal ligament is double than of the attached gingiva.99,102,26 Such active remodeling is necessary in a scenario where intense activity occurs, and none functional compromising is allowed. At the same time that many collagen fibers are destroyed, many others are generated. Recent evidences suggest that such intense activity is possible because of the progenitor pluripotent steam cells supply. Such cells can also work as a reserve for cellular differentiation and potential reconstitution of the tissues affected by periodontal diseases.96,97,29 Such cellular differentiation is mediated by cytosines present since the dental eruption stage.124,125,36,56,57 Such cytosines are also identified in the inflammatory process after orthodontic movement,1,72,118,12 promoting a programmed bone remodeling.43 This potential of response tends to decrease with aging,86 what changes the expectation of orthodontic results in adult patients.104 If orthodontic force is applied in an ankylosed tooth, which does not present periodontal ligament, bone remodeling around it is very limited. That is the same pattern of response induced on mini-implants, for temporary orthodontic anchorage.37
10. Laminate Alveolar Bone
- It is a specialized calcified connective tissue, dental-dependent, supported by trabecular alveolar bone, which is surrounded by the maxillary and mandibular cortical bones. Therefore, trabecular alveolar bone is recovered by laminate alveolar bone inside of the dental sockets, and surrounded by the bone cortices of the maxillary bones.89 The alveolar bone and the cortices converge and are fused in the alveolar crests. The dental insertion system provided by the periodontal ligament is functionally adapted to the masticatory forces and to the contact between adjacent teeth.The progenitor cells of the alveolar bone, with ectomesenchimal origin, are the osteoblasts. Osteoblasts make the bone matrix (osteoid), constituted by collagen fibers, glycoproteins and proteoglycans119 and mineralized by Calcium and Phosphate ions. Such deposition produces hydroxyapatite. Harvers´s channels allow the nutrition of osteocytes, cells that are locked in the mineralized bone and that communicate with periosteal osteoblasts. The “internal” surface of the alveolar bone is covered by endosteum, tissue that presents similar features of the periosteum.The laminate alveolar bone is specialized as Cementum and periodontal ligament. It also works for dental support. Its blood and lymphatic supply and its innervation are provided by the periodontal ligament throughout Volkman´s channels, among perpendicular attachments of Sharpey’s collagen fibers. The Havers´ channels of the alveolar bone communicate with the Volkman´s channels of the laminate alveolar bone, allowing instantaneous integration among bone, periodontal ligament and Cementum.
|
- Alveolar bone, laminate alveolar bone and periodontal ligament respond to the functional masticatory demand.8 In order to have such dynamic remodeling, continuously recycling of the Haversian system occurs. Bone suffers continuous resorption by osteoclasts and, at the same time, is neo-formed by osteoblasts.The alveolar bone is trabecular (under lesser functional demand) in interproximal regions and it is compact (under greater functional demand) in the vestibular and palatine/lingual surfaces of the teeth, fusing with the laminate alveolar bone. In these later regions, there is also a thickness difference: the bone is thicker in the vestibular and palatine/lingual surfaces in the molars region and thinner in the anterior teeth region. The functional load imposed by antagonist teeth is not limited to the centric static position, but is also related to mandibular excursion movements. As clinical consequences, the vestibular and palatine/lingual laminae of the posterior teeth offer greater resistance to orthodontic movements.87 Dental displacement is easier when the teeth moved in the “antero-posterior corridors” of the osseous dental arch. When non-controlled orthodontic forces are applied in the vestibular direction (for example, excessive palatine expansion), the bone laminae may suffer resorption. Bone fenestration or dehiscence may occur. The same happens in undesirable vestibular projection of the anterior teeth,123 during orthodontic leveling and alignment mechanics. In lower incisors bone fenestration or dehiscence mainly occurs due to disrespect to negative arch discrepancy and consequent vestibular flaring. Since bone remodeling is a dynamic, physiologic and natural process, orthodontic movement is well predictable, if well controlled. Orthodontic movement provokes inflammation in the periodontal ligament and increases its blood supply. Therefore, with the orthodontic activation occurs: 1) pain (in the first 3 to 5 days), 2) increase of the pericementary space (swallowing in the periodontal ligament) and, 3) increase in the local temperature (theoretical supposition, hard to be assessed). Such inflammatory process is aseptical in periodontally health patients and, if well planned, releases a desired and highly sophisticated biochemical process of bone deposition and resorption.62 In this process occurs bone deposition in the tension side (side “behind” the related orthodontic movement) and resorption in the pressure side (side “ahead” of the related orthodontic movement). Osteoblasts make the deposition line and osteoclasts make the resorption line. During orthodontic bone resorption, the osteoclastic action provokes the detachment of the periodontal ligament of the bone and dental anchorage in the socket is partially lost. Bone deposition in the endosteal side of such site aims to counterbalance such resorption. In the other side of the orthodontic movement, where bone deposition takes place, mineralization of the osteoid collagen matrix allows that Sharpey´s fibers are incorporated into the neo bone, promoting new periodontal insertion. In this site there is no resorption process in the endosteal side. When the periodontium of the teeth submitted to orthodontic movement is normal, good “bone balance” is achieved, and the amount of neo bone built in the deposition side equalizes the bone deficit between resorption and deposition in the resorption side.Ontogenetically, the cells which allow tissue adaptation to orthodontic forces are present since the first stages of dental formation. The normal process of eruption also requires bone deposition (and necessarily oestoblastogenesis) and bone resorption (and necessarily osteoclastogenesis).25,41 Sialoproteins and osteocalcins have been identified in the process of dental mineralization and in orthodontic movements.24,27 Proteinases have been identified in the process of bone resorption during dental formation and also in orthodontic movements.39,117,102,58,41Therefore, theoretically, orthodontic movements follow naturally developed physiologic patterns of dental eruption, and bone remodeling is naturally mediated by periodontal tissues.67,66 Scientifically pertinent, the biochemical comparison between dental eruption and orthodontic movements is still an ongoing research area.126,41
11. Conclusions
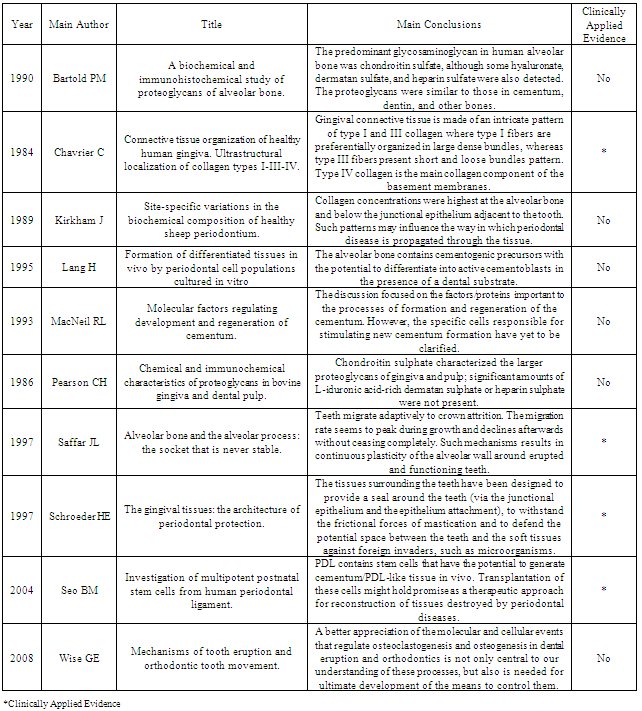
- Integration between the specialties Orthodontics and Periodontics, especially in adult patients, becomes crucial. In the table 01 the main results of the most scientifically relevant articles of the present paper are summoned. Such synergy in the modern and serious dental practice is fundamental, since favorable orthodontic movement is only achieved if the periodontal status of the patient is satisfactory. In this article we have described in a succinct way the main anatomic, histologic, physiologic, pathologic and therapeutic aspects of such integration, allowing basic knowledge for who wants to provide the best to the patients in these both areas.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML