-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Stomatological Research
2012; 1(1): 1-5
doi: 10.5923/j.ijsr.20120101.01
An Unusual Finding of Proliferative Periostitis in a Child: a Case Report
Saeed Asgary 1, Mohammad Jafar Eghbal 2, Sara Ehsani 3, Behnam Eslami 4
1Iranian Center for Endodontic Research, Dental Research Center, Shahid Beheshti University of Medical Sciences, Tehran, 1983963113, Iran
2Dental Research Center, Iranian Center for Endodontic Research, Shahid Beheshti University of Medical Sciences, Tehran, 1983963113, Iran
3Dental Research Center, Shahid Beheshti University of Medical Sciences, Tehran, 1983963113, Iran
4Department of Oral Medicine, Infection, and Immunity, Harvard School of Dental Medicine, Boston, MA, USA
Correspondence to: Saeed Asgary , Iranian Center for Endodontic Research, Dental Research Center, Shahid Beheshti University of Medical Sciences, Tehran, 1983963113, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Proliferative periostitis is a pathologic lesion that displays an osteo-productive and proliferative inflammatory response of the periosteum to infection or other irritation. This lesion is a form of chronic osteomyelitis that is often asymptomatic, occurring primarily in children, and found only in the mandible. The lesion can be odontogenic or non-odontogenic in nature. A 12 year-old boy presented with an unusual odontogenic proliferative periostitis that originated from the lower left first molar, however, the radiographic radiolucent area and proliferative response were discovered at the apices of the lower left second molar. The periostitis was treated by single-visit non-surgical endodontic treatment of lower left first molar without antibiotic therapy. The patient has been recalled regularly; the lesion had significantly reduced in size 3-months postoperatively. Extraoral symmetry occurred at approximately one year recall. At the last visit, 2 years after initial treatment, no problems or signs of complications have occurred; the radiographic examination revealed complete resolution of the apical lesion and apical closure of the lower left second molar. Odontogenic proliferative periostitis can be observed at the adjacent normal tooth. Besides, this case demonstrates that non-surgical endodontics is a viable treatment option for management of odontogenic proliferative periostitis.
Keywords: Endodontics, Proliferative Periostitis, Odontogenic
Article Outline
1. Introduction
- Proliferative periostitis in long bones was first described by Garrè in1983[1]. The first documented case in dental literature was reported in 1941[2]. The condition is characterized by new bone formation on the periphery of the cortex over an infected area of spongiosa, which is a response of the inner surface of the periosteum to stimulation by a low-grade infection that has spread through the bone and penetrated the cortex. Staphylococci and streptococci are the microorganisms most commonly found in the area. Periapical odontogenic infection is a frequent cause of proliferative periostitis of the jaws, however, the condition is a relatively uncommon dental complication[3]. Wood suggests that for the lesion to develop, the following combination of circumstances must coexist[3]:1- The periosteum must possess a high osteoblastic activity (which is the case in young individuals).2- A chronic infection must exist.3- A fine balance must be maintained between the resistance of the host body and the virulence of microorganisms so that a mild, chronic infection can continue; invasive enough to stimulate new periosteal bone formation while not severe enough to induce bone resorption.Eversole noted that the entity occurs exclusively in children (average age of ~11 years][4]. Proliferative periostitis typically presenting unilaterally in the posterior two thirds of the mandible[5,6]. A male predominance of 1.4 to 1 has been reported. Sporadic cases had been reported in patients in their third decades and in infants[7]. An infected mandibular first permanent molar is the most common etiologic factor[3,8]. There may be a history of pain caused by the odontogenic infection before the swelling develops at the inferior border or other peripheries of the mandible. The swelling typically presents unilaterally in the posterior two thirds of the mandible[5,6] and the most frequent site is the inferior border of the mandible below the fist molar. The maxilla is seldom affected. The condition is characteristically solitary, however, a case with simultaneous lesions in all four quadrants has been reported[3].The swelling usually causes moderate facial asymmetry, and is asymptomatic, with no associated tenderness. With the establishment of the chronic infection beneath the periosteum, new bone is laid down and the swelling soon becomes hard. The mass is bony hard in palpation and the appearance of the overlying skin or mucosa is typically normal but it may be moderately inflamed.Although the length and depth of bone deposits vary, the swelling is characteristically convex. The size may range from about 2 centimetres to involvement of whole length of mandibular body on the affected side. Fever and leukocytosis are occasional features[3].Unlike other forms of osteomyelitis, usually hyperthermia is absent and the blood sedimentation rate is normal. Also, there is no distinct increase in the white blood cell count or alkaline phosphatase values[9]. On radiographic examination, a smoothly contoured, moderately convex radiopaque lesion can be seen extending from the cortex of the jaw. The space between this new, thin shell of bone and the pre-existing cortex may be quite radiolucent without images of trabeculae. With time, an alternating radiolucent and radiopaque, laminated appearance may be seen, which makes the characteristic radiographic feature of this disease in the occlusal view, known as the "onion skin" appearance[9-11]. The appearance indicates parallel new reactive bone formation which expands the surface of the altered bone. In most reported cases, the adjacent jawbone appears normal on the radiograph, but in some cases, the condition may be accompanied by osteolytic or osteosclerotic osteomyelitis changes and small sequestra may be noticed. Periapical radiographs usually reveal a radiolucent area at the apex of the involved carious tooth[8,12]. Histological examinations show proliferation of new reactive bone on the bone cortex and beneath the periosteum with fibro-osseous pattern and minimal vascular space. One of the characteristics of this new bone is the presence of osteoblastic rimming and osteoid tissue. Also, in the existing fibrous connective tissue the infiltration of chronic inflammatory cells can be seen[13]. This pathologic entity has been described under different clinical terms including chronic diffuse sclerosing osteomyelitis, chronic osteomyelitis with proliferative periostitis, Garrè's osteomyelitis, chronic non-suppurative sclerosing osteitis, osteomyelitis sicca, and periostitis ossificans[13]. The term Garrè’s osteomyelitis has almost completely been rejected by most medical pathologists but still enjoys acceptance in dental field[14].The general treatment consensus is to extract the offending tooth; however, root canal treatment (RCT) has also been proposed as a viable treatment option for proliferative periostitis[6,9]. In this article, a case of proliferative periostitis is reported radiographically correlated with the lower left second molar. The lesion was completely resolved by one - visit non-surgical endodontic treatment of lower left first molar (which was considered the origin of the lesion). Up to our knowledge, this is a unique case and has not been reported before.
2. Case Report
- A 12-year-old boy was referred for evaluation of a swelling on the left posterior mandibular region, which had come to notice a few months ago. His medical history revealed no present or past record of disease or problems. The patient and his parents reported no history of intraoral or extraoral trauma. He had had no previous experience of dental treatment. In clinical observation, the patient had an apparently normal general and local growth and development. Extraoral palpation of the lesion revealed a hard, non-fluctuant, smooth swelling, with no associated tenderness. The size of the palpable swelling was approximately 3×4 centimetres. The appearance and colour of the facial skin was normal and lymphatic nodes showed no signs of swelling on visual and tactile examination. Intraoral examination revealed a large mass on the mucobuccal area of left mandibular molars region; the overlying mucosa had normal colour and appearance. The lower left second molar was sound; however, the lower left first molar had a deep and extensive class I carious cavity. None of the teeth in the region displayed mobility.The periapical radiographic examination demonstrated a large poorly defined radiolucent lesion around the apices of the lower left second molar (Figure 1); the apices were both immature. Surprisingly, the mandibular first molar showed only mild PDL widening around the tooth and the lamina dura at the apical part of the distal root was blended into the radiolusent lesion. Occlusal radiographic view illustrated a series of radiopaque lines, resulted by formation of periosteal new bone and the characteristic "onion skin" appearance in the second molar region (Figure 2). The patient reported no fever and all blood tests including complete blood count (CBC), Wright, Vidal, and Tuberculin tests were within normal range. Panoramic, skull and chest X-rays were ordered to rule out other possible diseases (Figures 3-4). The diagnosis was set as proliferative periostitis due to pulp/periapical infection. Despite the fact that in all radiographic examinations, the mandibular second molar appeared to be the cause of the condition, the deep caries in the mandibular first molar convinced us to consider this tooth as the origin of the lesion.
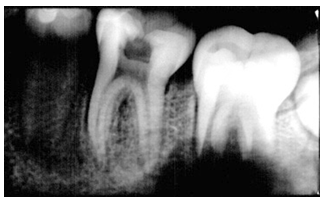 | Figure 1. Pre-treatment periapical radiograph of mandibular first and second molars shows lytic foci in association with permanent second molar |
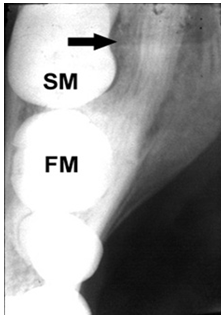 | Figure 2. Pretreatment occlusal radiograph of dense paracortical mass (left side of mandible, FM: first molar, SM: second molar, black arrow: center of the lesion) |
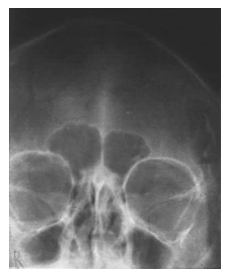 | Figure 3. Preoperative skull Waters view |
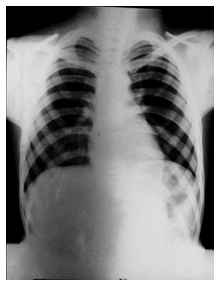 | Figure 4. Chest x-ray; radiographic examination of the lungs, heart, large arteries, ribs, and diaphragm shows normal appearance |
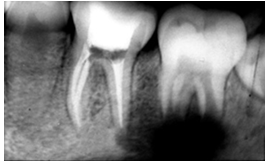 | Figure 5. Mandibular left first molar at completion of endodontic treatment |
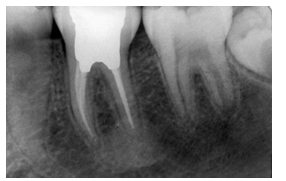 | Figure 6. One year after endodontic treatment; periapical radiolucent area is completely filled in with normal bone |
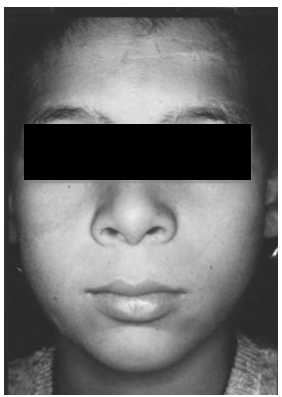 | Figure 7. Extraoral symmetry after one year |
3. Discussion
- Bone formation, as a result of periosteal reaction, occurs secondary to a number of bony pathosis and is not unique to the jawbones. In the orthopaedics, proliferative periostitis of the tibia is a well-known pathology and usually involves the anterior aspect of the tibia[7]. A same appearance can be found in osteomyelitis, trauma, several neoplastic diseases, fibro-osseous lesions and proliferative periostitis of Garrè [15-17]. In this case, the diagnosis of proliferative periostitis was confirmed after performing the serologic, immunologic and complete blood tests, in addition to radiographic examination of skull, jaws, and chest.However, the presence of proliferative periostitis surrounding an adjacent tooth is rare to say the least. In previous reported cases, the lesions were found surrounding the offending tooth[6,9, 18-20]. In the present case, the distal root apex of lower left first molar was connected to the periapical lesion of adjacent second molar, and as the first molar suffered from a necrotic pulp, the lesion was undoubtedly caused by the first molar. Non-surgical root canal treatment can effectively eliminate etiological factors (i.e. microorganisms and their by-products) and therefore other treatments or adjunct antibiotic therapy was not deemed necessary[21]. The previous school of thought advocated surgical re-contouring of bone[22]; however, more recent evidence demonstrates that after good non-surgical root canal therapy, bone remodelling would take place physiologically[6] as the bone is re-contoured by the functional forces[3]. Pulp exposures from caries can cause pulpal infection which eventually results in periapical disease. The bacteria and their inflammatory by-products pass through spongeous and cortical bone and reach beneath the periosteum. They stimulate periosteal osteoblasts, leading to apposition of new bone. With periodic irritation/stimulation, this process repeats several times and new bone is added to the previous bone in layers. Radiographic examination shows bony laminations parallel to each other and to the cortical surface of the involved bone[23-25]. Appropriate radiographic angulation may bring out the radiolucent zone of soft tissue between the bony cortex and the newly formed laminations. This characteristic phenomenon is described as an "onion skin appearance" in occlusal radiography[9-11]. Since non-surgical endodontic treatment removes the etiologic factors, it allows osteoblastic activity to be replaced by osteoclastic activity. After a certain period of time, the bone surface will remodel to its condition before the disease. The majority of articles have reported approximately one year time span for complete healing; this is consistent with our clinical finding[9,26-28].
4. Conclusions
- The epicentre of the odontogenic proliferative periostitis can be seen at the adjacent normal tooth. Care must be taken to set the correct treatment plan. The results of this case study show that one-visit non-surgical endodontic management without antibiotic therapy can be the treatment of choice in odontogenic proliferative periostitis.
ACKNOWLEDGMENTS
- The authors wish to thank staff of Iranian Center for Endodontic Research as well as Iran Center for Dental Research who assisted in the preparation of this report.
Conflict of Interest
- The author(s) declare no conflict of interests
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML