-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Prevention and Treatment
p-ISSN: 2167-728X e-ISSN: 2167-7298
2023; 11(1): 1-7
doi:10.5923/j.ijpt.20231101.01
Received: Feb. 14, 2023; Accepted: Mar. 6, 2023; Published: Mar. 15, 2023

Determinants of Soil Transmitted Helminthiasis Control Practices at the Household Level in Bondo Sub County in Siaya County
Allan Ogomo Amulabu1, Dr. Alice Lakati2, Dr. Josephat Nyagero2
1AMREF International University, Nairobi, Kenya
2Siaya County Health Office, Siaya, Kenya
Correspondence to: Allan Ogomo Amulabu, AMREF International University, Nairobi, Kenya.
| Email: |  |
Copyright © 2023 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Soil-transmitted helminths (STH) refer to the intestinal worms infecting humans that are transmitted through contaminated soil (Cdc; 2022). STH infections are common infections worldwide and affect the poorest communities, especially pre-school and school aged children. WHO’s strategy for control of STH infections is through the periodic mass drug administration treatment with dewormers. Objective: The general objective of this study is to find out the determinants of soil transmitted helminthiasis (STH) control practices at the household level. Methods: This was a cross-sectional study conducted in Bondo subcounty, Siaya county in Kenya. 530 households were randomly selected and include in the study. A Questionnaire was administered to respondents at the household level and an FGD guide was also used to gather more insights on the subject. Results: The result of this study indicates that 28.3% of respondents were able to correctly identify at least one of the three types of STH. 40.3% and 78.4% of respondents were able to correctly identify transmission routes and control practices respectively. The most reported practice in the control of STH was deworming (38.1%); household factors found to be significantly associated with STH control practices were as follows, Maternal education, Mother’s Occupation, Participation in previous programme, Household income, availability of handwashing facilities. Conclusions: The knowledge and practices of STH control was found to be low despite the socio-economic status of the study area being medium. Most homesteads reported to have above minimum wage income, high literacy level, adults in gainful income generating activities, latrine in the homestead, houses of concrete finished floors and good access to water. Our findings suggest that to effectively develop an STH control program, maternal, household socio-economic and WASH factors need to be put into consideration.
Keywords: Determinants, Soil Transmitted Helminthiasis, Control, Household
Cite this paper: Allan Ogomo Amulabu, Dr. Alice Lakati, Dr. Josephat Nyagero, Determinants of Soil Transmitted Helminthiasis Control Practices at the Household Level in Bondo Sub County in Siaya County, International Journal of Prevention and Treatment, Vol. 11 No. 1, 2023, pp. 1-7. doi: 10.5923/j.ijpt.20231101.01.
1. Background
- Soil-transmitted helminths (STH) refer to the intestinal worms infecting humans that are transmitted through contaminated soil (Cdc; 2022). They are transmitted by eggs present in human feces which in turn contaminate soil in areas where sanitation is poor (Who; 2022). The main species of STH are the roundworm (Ascaris lumbricoides), the whip- worm (Trichuris trichiura) and hookworms (Necator americanus and Ancylostoma duodenale). (Cdc; 2022). According to WHO, more than 1.5 billion people, or 24% of the world’s population, are infected with soil-transmitted helminth infections world- wide. Almost 70% of STH infections occur in Asia with a high proportion of total individuals infected with one or more STH residing in the People’s Republic of China (18%) and India (21%). In sub–Saharan Africa, the three most populous nations (Nigeria, Ethiopia, Congo DRC) in total account for only 8% of global STH infections. In Kenya STH infection is prevalent in 66 sub-counties endemic for both STH in four regions (Western, Nyanza, Rift Valley and Coast) (Mwandawiro et al.; 2019). In 2011, a National School Health Policy and National Multi-Year Strategic Plan for the Control of NTDs were developed and called for treatment to be administered to all SACs, including those out of school, based on the prevalence and in- tensity of STH and schistosome infections in the 66. Research shows that after five years of MDA implementation, Coast Region had the highest reduction in STH infection at 87.6% followed by western region which had 60.6% then, Nyanza had 59.4% and only managed 27.5% reduction and remains the region that harbors majority of STH infections. Additionally, the report indicate that after five years of MDA, four counties (Kericho, Kisii, Narok and Vihiga) still had prevalence of any STH infections ranging between 20% and < 50%, another four counties (Bomet, Busia, Homa Bay and Nyamira) had prevalence ranging between 10% and < 20%, while seven counties (Bungoma, Kakamega, Kilifi, Kisumu, Kwale, Migori and Mombasa) had their prevalence range between 1% and < 10%, and only Taita Taveta County recorded prevalence below 1% (Mwandawiro et al.; 2019), (Ng’ang’a et al.; 2016).
2. Methods
- This was a cross-sectional study conducted in Bondo subcounty, Siaya county in Kenya. 530 households were randomly selected and include in the study. A Questionnaire was administered to respondents at the household level and an FGD guide was also used to gather more insights on the subject. It was the most preferred data collection instrument because of its anonymity. The questions were closed and coded for easier analysis. The instrument was key in collecting data on the determinants affecting STH control at the household level and at the school level as well as collecting data from key govern- ment officials on the existing national efforts employed in STH control. The responses were coded and no data identifying par- ticipants (names) were collected. All households in Bondo Sub County with headed by an adult who has a sound mind of ages 18 – 59 and 60 years or older were included in the study. To systematically explore the determinants of STH control practices among the SAC, the study aimed to collect data on the following variables (occupation of care giver, type of floor, household income, access to water, access to sanitation, hygiene practice, knowledge of STH infections, knowledge of STH control measures and deworming). Data collected were summarized in frequencies, tables for illustrative purposes. At univariate level Chi square tests were performed to identify the statistical significance of the relationship between the variables.Variables which were significant at univariate analysis were taken further for multi-variate analysis using multiple regression to determine the strength of association between the independent variables and STH control practices. After finding the strength of association at multi variate level, bi variate analysis using logistic regression were used to determine statistical association exists between independent variable and STH control practices.
3. Results
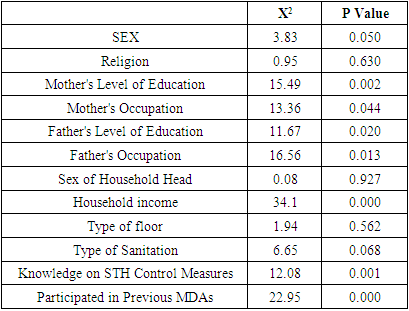
- Socio-demographic characteristics of respondents: A total of 538 households were enrolled in this study. 74.2% (n=399) of the respondents were female and 25.8% (n=139) were male. Most of the respondents, 94.2% (n=507), were Christians, 5.2% (n=25) confessed to be members of the traditional African religions and 0.6% (n=5) indicated they were Muslims. 93.7% (n=504) of the respondents indicated that their households had a mother present while 76.0% (n=409) of the respondents indicated that their households had a father present. 4.6% (n=25) of the households reported having mothers without any formal education, most mothers, 54.8% (n=295) had primary school level education, 27.9% (n=150) had a high school level education and only 6.3% (n=34) had a tertiary level education. Only 1.5% (n=8) of the households reported having fathers who had no formal education, most fathers, 36.4% (n=196) had primary school level education, a further 27.9% (n=150) of them had a high school level education and 10.6% (n=57) of fathers were reported to have a tertiary level education. 74.0% (n=398) of the households were male-headed households. Factors that influence transmission of Soil Transmitted Helminths (STH) at the household level in Bondo: A bivariate analysis was carried out to determine the factors associated with STH Control practices at household level in Bondo. Seven variables were found to be associated with STH Control practices at household level at bivariate analysis. They included: household income (X2=34.10); mother’s level of education (X2=15.49); father’s level of education (X2=11.69); mother’s occupation (X2=13.36); father’s occupation (X2=16.56); respondents’ knowledge of STH (X2=12.08); and participation in previous STH control campaigns (X2=22.95).
|
4. Discussions
- This study is important towards efforts in the control STH in a high prevalence. It established context-specific evidence on determinants of STH control practices at household level in Bondo Sub County in Kenya. This information is critical for policy makers in Kenya to improve control strategies of STH. Socio-demographic characteristics of respondents Most households had parents with at least primary school level formal education. This indicates that the literacy level in this area is high. This high literacy level can be associated to the high proportion of people in gainful income generating activities in the study area. The high proportion of people in occupation can be attributed to the general lifestyle in the community. Most of the homes were found to have concrete floors and most homes had income above the minimum wage in Kenya with most respondents indicating that they never walked bare feet. A previous study by Anuonobi and others (2019), identified socio- economic factors such as poverty, lack of portable water, occupation, as determinants of STH distribution in endemic areas. This sentiment was a build up to another study by Halpenny and others (2013) who concluded that low relative household wealth and maternal education and infrequent latrine use were major contributing factor in Ascaris reinfection. Benjamin-Chung et al. (2019) concluded that in low resource settings where the floors of houses were not finished with concrete, there was an increase in the transmission of soil transmitted helminth. In the present study we note that most of the households had homes finished with concrete floors and this could explain why there is reported low level of STH control practice as residents appear to be of a relatively medium socio-economic status. Addition- ally, In the present study type of occupation was not a significant factor in the control efforts of STH at the household level.
|
5. Conclusions
- In our study area, the knowledge and practices of STH control was found to be low. The socio-economic status of the study area was found to be medium with most homesteads reported to have above minimum wage income, high literacy level, adults in gainful income generating activities; latrine in the homestead, houses of concrete finished floors and accessing water in less than 30 minutes. These important findings point to the need to consider developing STH control messaging that are context specific to such communities. We observed that STH control practices were generally associated with maternal level of education, maternal occupation, availability of handwashing facilities, sharing of la- trines, previous participation in STH programme and household income. Our results suggest that to effectively develop an STH control program, the following factors will need to be put into consideration: maternal education; maternal occupation; house- hold income; availability of water and handwashing facilities and availability of latrines at the household level.
ACKNOWLEDGEMENTS
- I am grateful to the County Government of Siaya department of health and all the participants who made this study possible. Source of funding: The cost of this study was covered by the principal author, there were no external funding.Conflict of interest: The authors had no conflicting interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML