-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Prevention and Treatment
p-ISSN: 2167-728X e-ISSN: 2167-7298
2019; 8(2): 46-51
doi:10.5923/j.ijpt.20190802.03

Prevalence of Sarcopenia among Hemodialysis Patients in a University Hospital, Cairo, Egypt
Doaa SE Zaky, Amany M. Abdallah
Department of internal medicine, Al-Zahraa University Hospital, Al-Azhar University, Cairo, Egypt
Correspondence to: Doaa SE Zaky, Department of internal medicine, Al-Zahraa University Hospital, Al-Azhar University, Cairo, Egypt.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background/aims: Sarcopenia is a generalized loss of skeletal muscle mass combined with reduced strength and/or physical performance that has been associated with adverse clinical outcome in older individuals. The accelerated process of protein catabolism in patients with end stage renal disease (ESRD) on maintenance hemodialysis (HD) can lead to sarcopenia. The aim of the present study was to assess the prevalence of sarcopenia and its correlates among adult patients with ESRD on maintenance HD. Methods: This was a prospective cross-sectional study carried out for 1 month in dialysis unite of the internal medicine ward in a University hospital in Cairo, Egypt. Patients were diagnosed for sarcopenia by the European Working Group on Sarcopenia (EWGS). Skeletal muscle mass was measured using bioimpedance analysis (BIA), muscle strength measured by a handheld dynamometer and physical performance measured by 4-meter gait speed test. Different clinical and pre-dialysis biochemical parameters were collected. Results: A total of 37 patients on maintenance HD were participated in the study (median age of 50 years and interquartile range (IQR) between 35 to 60.5 years; 54.1% were males). The prevalence of sarcopenia in the study participant was 35.1% (45% in men and 23.5% in women). Skeletal muscle index (SMI) showed significant positive correlation with age (r=0.406, P= 0.013) and BMI (r=0.631 and P< 0.001). Conclusions: Sarcopenia was highly prevalent among ESRD patients on maintenance HD. Young age and low BMI were among factors that increased the risk of sarcopenia. Screening for sarcopenia in the HD sitting is mandatory to increase clinical diagnoses and ultimately, care for people with sarcopenia.
Keywords: Sarcopenia, Skeletal muscle mass, Hemodialysis
Cite this paper: Doaa SE Zaky, Amany M. Abdallah, Prevalence of Sarcopenia among Hemodialysis Patients in a University Hospital, Cairo, Egypt, International Journal of Prevention and Treatment, Vol. 8 No. 2, 2019, pp. 46-51. doi: 10.5923/j.ijpt.20190802.03.
Article Outline
1. Introduction
- Sarcopenia is a clinical condition characterized by adverse changes in the skeletal muscle that occur across the lifetime. It can be defined by low levels of measures for three parameters: (1) muscle strength, (2) muscle quantity/quality and (3) physical performance as an indicator of severity [1]. Sarcopenia has long been associated with ageing and older people but can also occur earlier in life [2]. Primary sarcopenia is considered when no specific cause is evident and secondary sarcopenia when causal factors other than or in addition to ageing are evident [3]. Other contributing causes beyond ageing include inflammatory processes, malignancy, organ failure, physical inactivity and inadequate intake of energy or protein secondary to anorexia, malabsorption or limited ability to eat [4]. Sarcopenia is an emerging serious public health concern due to its impact on human health as well as health care system. As regard to human health, sarcopenia have multiple clinical consequences as it is associated with cardiac diseases [5], respiratory diseases [6], cognitive impairment [7], mobility disorders with increased risk of falls and fractures [8,9] and lowered quality of life [10]. In addition, sarcopenia is also having a great financial impact on the healthcare systems due to increased risk for hospitalization with higher hospital costs in sarcopenic patients [11]. Sarcopenia is common in chronic kidney disease (CKD) and reported as key risk factor for adverse outcome in patients on renal replacement therapy [12,13]. Multiple factors are contributing to the impairment of skeletal muscle mass and function thus predisposing CKD patients to sarcopenia. Dyslipidemia [14], insulin resistance [15] and metabolic acidosis [16] are among metabolic factors and anorexia, altered taste sensation, weight loss and homeostatic disturbances of energy and protein balances [17] are among nutritional factors that predispose to sarcopenia in CKD patients.Hemodialysis (HD) in end stage renal disease (ESRD) has been associated with acceleration of the symptoms of sarcopenia [18,19]. Sarcopenia in the sitting of dialysis was contributed to dialysis procedure, intercurrent illness, hyper-catabolism and reduced anabolism, and chronic inflammatory state [13]. The prevalence of sarcopenia in patients with CKD ranges from 5% to 37%, depending on the adopted definition and stage of renal impairment [20]. However, conflicting results exist concerning its precise prevalence in dialysis patients. Sarcopenia has been reported in about 20% of European patients undergoing dialysis [21], whereas its prevalence in Asian patients undergoing hemodialysis (HD) was 37.0% in men and 29.3% in women [22] and 13.7% in both sex in another study also in Asia [23]. Sarcopenia has been overlooked and undertreated in mainstream practice of HD patients. Screening for sarcopenia among those patients could potentially allow for early interventions with protective measures that may retard its progression. There are few researches which studied the prevalence of sarcopenia among EDRD patients in maintenance HD in Arab world and to our knowledge; this is the first one dealing with this issue in Egypt. The aim of the present study was to assess the prevalence and correlates of sarcopenia in maintenance HD patients to enhance awareness and care for sarcopenia in those patients.
2. Patients and Methods
2.1. Study Participants
- This was a prospective cross-sectional study, conducted at Al-Zahraa University Hospital, Cairo, Egypt at the dialysis unit of internal medicine ward. The unit contains 22 HD machine and functions as an open unit to provides support to CKD patients with ESRD. The study was carried out over a period of one month (May 2019). The study includes 37 patients with ESRD aged ≥ 18 years old on regular HD three-times a week for at least six months. The sample size was calculated by using equation in the Raosoft software package according to annual flow on dialysis units with accepted margin of error 5% and the confidence level 95%. Informed consent was obtained from all study participants in advance. All procedures were performed in accordance with the guidelines in the Declaration of Helsinki. Patients with acute kidney injury, active malignancy or acute sever infection or those with inability to walk or use their hands were excluded from the study. All patients were subjected to a standardized questionnaire including demographic information, primary cause of CKD, duration of dialysis and comorbidities. Physical examination was done with special emphasis to anthropometric measurement in the form of height/m and weight/kg. Body mass index (BMI) was calculated using the equation; BMI = weight (kg) divided by the square of the height (m).
2.2. Assessment of Sarcopenia
- Three parameters were assessed to diagnose sarcopenia; muscle strength, skeletal muscle mass (SMM) and physical performance [1]. Muscle strength was measured by grip strength using calibrated handheld dynamometer [24]. Grip strength correlates moderately with strength in other body compartments, so it serves as a reliable test for muscle strength [25]. The test was administered on the dominant hand and each patient was asked to squeeze as hard as he/she can. Three trials were allowed with a pause of 10 to 20s between each trial to avoid excessive fatigue, and the maximum effect was recorded. Grip strength with cut of < 32 kg in men and < 22 kg in women was diagnosed as low muscle strength [26]. Bioelectrical impedance analysis (BIA) was used to estimate the SMM by Inbody Dial H20b Analyzer. Skeletal muscle index (SMI) was calculated by SMM in kg adjusted for the squared height (SM/height2, kg/m2). The cut-off thresholds for SMI were 9.2 kg/m2 and 7.4 kg/m2 in males and females, respectively [26]. Physical performance (whole-body function related to locomotion) was measured by 4-meter gait speed test with cutoff value ≤0.8 m/s as an indicator of low physical performance [27]. Sarcopenia was diagnosed according to EWGS [1] by; low muscle strength (probable sarcopenia) that confirmed by low muscle mass. When low muscle strength plus low muscle mass were accompanied by low physical performance, sarcopenia is considered severe.
2.3. Laboratory Investigations
- Biochemical and haematological tests were collected pre-dialysis following overnight fasting. Serum albumin, total cholesterol, phosphorus, calcium, hemoglobin, and ferritin were performed using standard laboratory methods.
2.4. Statistical Analysis
- Data collected was reviewed, coded and statistically analyzed using IBM SPSS statistics (V. 25.0, IBM Corp., USA, 2017-2018). Data were expressed as median and interquartile range (IQR) for quantitative non-parametric measures in addition to both number and percentage for categorized data. Comparison between two independent groups for non-parametric data was done by using Wilcoxon Rank Sum test. Ranked Sperman correlation test was applied to study the possible association between two variables among each group for non-parameteric data. Chi-square test was used to study the association between each 2 variables or comparison between 2 independent groups as regards the categorized data. The probability of error at 0.05 was considered significant, while at 0.01 and 0.001 are highly significant.
3. Results
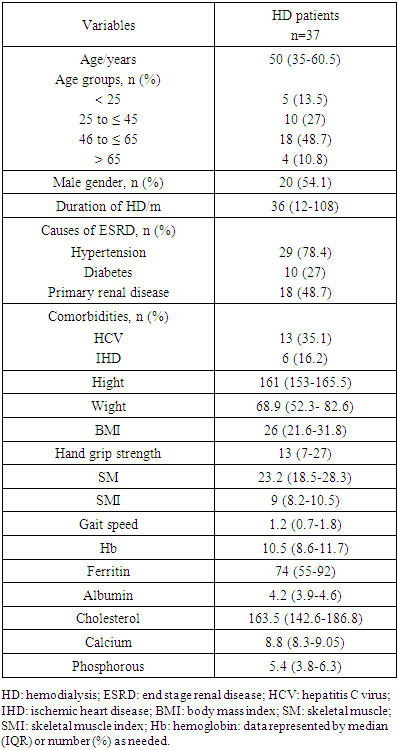
- A total of 37 patients on maintenance HD were met the selection criteria and included in the study. Table 1 shows the general characteristics of the HD patients. Their age ranged between 19 and 74 years with median age of 50 years and IQR from 35 to 60.5 years. Most of our patients were ≤ 65 years (89.2%) and non above 75 years old. Female gender constituted less than half (45.9%) of them. The median duration of dialysis was 3 years ranged between 6 and 240 months. Hypertension was the most common cause of ESRD in the study patients (78.4%) followed by primary renal diseases and diabetes (48.6% and 27% respectively). HCV was positive in about 35.2% of patients.The median value of hand grip strength was low (13) kg ranged between 3 to 38 kg and about 86.5% of patients had low grip strength according to the reference for male and female values. As regard to median SMI it was also low (9 kg/m²) in HD patients ranged between 4.5 to 19.1 kg/m² depending on the criterion used to index total-body muscle mass. However, the median value of gait speed was within the normal range 1.2 (0.7-1.8).
|
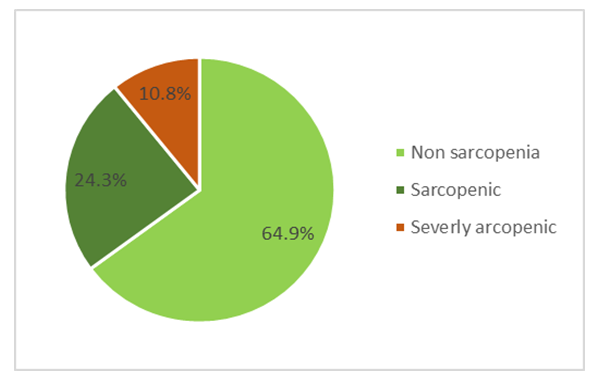 | Figure 1. Prevalence of sarcopenia among studied HD patients |
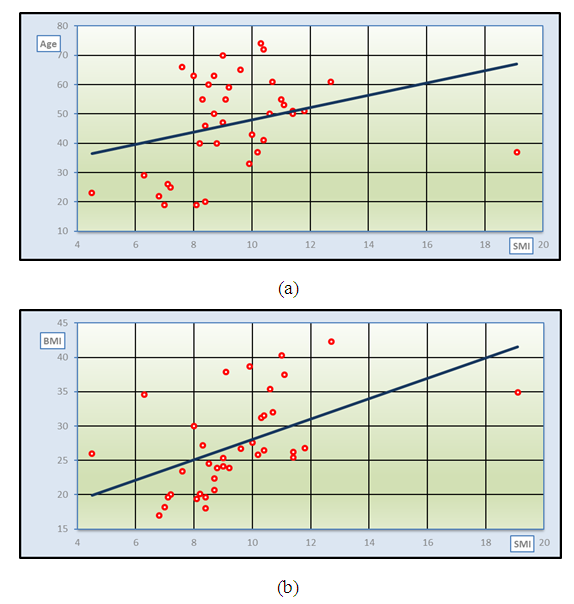 | Figure 2. Correlation between SMI with age (a) and BMI (b) in HD patients |
|
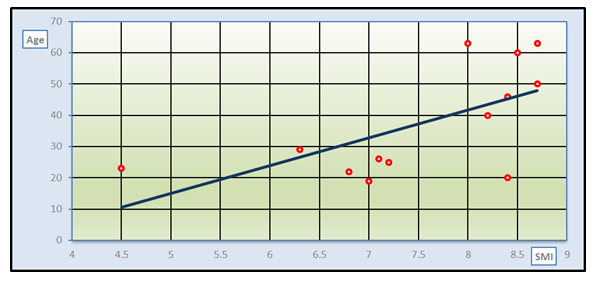 | Figure 3. Correlation between SMI and age in sarcopenic HD patients |
4. Discussion
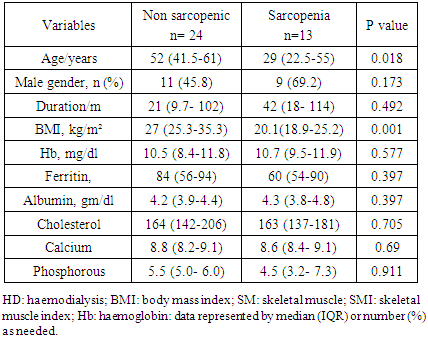
- This prospective cross-sectional study was conducted in 37 ESRD on maintenance HD to assess the prevalence of sarcopenia and its correlation with certain clinical and biochemical factors. In the present study; the prevalence of sarcopenia among HD patients was 35.1% (45% in men and 23.5% in women) according to the EWGS reference standard with 10.8% of patients with severe sarcopenia. This prevalence was comparable to earlier study by kim et al [22] in 95 maintenance HD patients over 50 years old (mean age 63.9 ± 10.0 years) which reported sarcopenia in 37.0% in men and 29.3% in women. Higher prevalence of sarcopenia (41%) were founded by Lamarca et al [28] that enrolled 102 maintenance HD patients of over 60 years old. Nearly the same prevalence (40%; 37% in males and 45% in females) was also reported recently with insignificant gender differences [29]. However, lower prevalence of sarcopenia was reported by Isoyama et al in a study that enrolled 330 incident dialysis patients and 20% of patients had sarcopenia [21]. Differences in prevalence of sarcopenia among HD patients may be related to age and gender [30] as well as the diagnostic tools used [31,32].Incidence of sarcopenia in HD patients was reported as 13.7% total incidence and 33.3% over 60 years that increased gradually with age and dialysis duration [23]. Contradictory to the previous data, our data that revealed significantly younger age in sarcopenic compared to non-sarcopenic patients (P<0.05) and positive correlation of SMI (as the main determinant of sarcopenia) with age. Young age in our results seems to be risk of sarcopenia in ESRD on regular HD. These contradictory results may contribute to CKD as a cause of sarcopenia rather than age as most of our patients were ≤ 65 years (89.2%). Moreover, primary renal insult is the cause of ESRD in about half of patients (48.6%). This data may raise the speculation that ESRD and HD in young age has more deleterious effect on muscle mass and function than in older age. Duration of HD in sarcopenic patients was higher than in non-sarcopenic patients in our study, however, it didn’t reach a significant value and didn’t correlate with SMI. Dialysis duration was closely related to the development of sarcopenia due to its association with several pathological states such as hormone imbalance, malnutrition, loss of ATP and glycogen, oxygen transportation disorder caused by anaemia, metabolic acidosis, electrolyte disturbance, lifestyle changes and muscle atrophy [18].Uremic sarcopenia is a phenomena of decreased muscle mass and force as a result of increased levels of uremic toxins. Sarcopenia in HD patients can be also attributed to multiple contributors such as increased levels of pro-inflammatory cytokines, insufficient protein intake, and insulin resistance. Elevated level of tumor necrosis factor-α (TNFα) in ESRD may also increase muscle wasting by activating nuclear factor-κβ pathway and attenuating insulin-stimulated protein synthesis [33].Low BMI is considered as another risk factor for sarcopenia in our study as sarcopenic had significantly lower BMI than non-sarcopenic HD patients. The development of sarcopenia was often closely related to malnutrition. Protein-energy wasting of CKD patients manifesting as the reduction of proteins in the circulating system and the loss of body weight and muscle mass [34]. However, other nutritional laboratory parameters as serum albumin and serum cholesterol had no significant differences between HD patients according to diagnosis of sarcopenia. The nutritional evaluation indices were insufficient in screening for sarcopenia among the elderly [35].Follow up of sarcopenia in HD patients was reported to be strongly associated with long-term mortality and cardiovascular events [36]. Moreover, low hand grip strength (one component of sarcopenia) can predicts all-cause mortality in HD patients [37]. In the present study most of the studied HD patients (86.5%) had low grip strength. In another study, among Japanese HD outpatients who were able to walk independently, slow usual gait speed and weak hand grip strength were significantly associated with CV events, independent of age, sex, HD duration, and medical history [38].Hyperphosphatemia is commonly seen in maintenance HD patients and is known as a risk of vascular calcification and cardiovascular death [39]. Although the study patients had high median phosphorous level, the serum phosphorus level of sarcopenic group was lower than that of non-sarcopenic group and the two groups showed no significant difference as regard to serum phosphorous.The possible reason is that high-protein diet is the main source of dietary phosphorus so, the reduction of food intake will certainly lead to the decrease of patients’ serum phosphorus, malnutrition and protein-energy consumption, that eventually lead to sarcopenia. Unmeasured confounding factors of sarcopenia as protein intake and physical activity were considered as limitation to this study as well as the small sample size due to its nature as a single-centre cross-sectional study. Finally, more studies on the prevalence of sarcopenia in young ESRD patients with maintenance HD is recommended.
5. Conclusions
- Sarcopenia was highly prevalent among patients with ESRD on maintenance HD. Hence; there is a need for raising awareness for the diagnosis of sarcopenia in the dialysis sitting to provide early nutritional intervention aiming to decrease personal, social and economic burdens of untreated sarcopenia. Low BMI was among factors that increased the risk of sarcopenia as well as younger age onset of ESRD that may have more deleterious effect on muscle mass and function in patients with HD. Further studies with larger sample size on young aged HD patients were needed to augment this result.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML