| [1] | Brosnan JT. 2003, Interorgan amino acid transport and its regulation. J Nutr 133 (6 Suppl 1): 2068S-2072S. |
| [2] | Newsholme P, Lima, Procopio MR, Pithon-Curi TC, Doi SQ, Bazotte RB, Curi R. 2003, Glutamine and glutamate as vital metabolites. Braz J Med Biol Res 36(2): 153-163. |
| [3] | Berg A, Rooyacker O, Norberg A, Wernerman J. 2005, Elimination kinetics of L-alanyl-L-glutamine in ICU patients. Amino Acids 29: 221-228. |
| [4] | Lacey JM, Wilmore DW. 1990, Is glutamine a conditionally essential amino acid?. Nutr Rev 48(8): 297-309. |
| [5] | Vinnars E, Hammarqvist F, von der Decken A, Wernerman J. 1990, Role of glutamine and its analogs in posttraumatic muscle protein and amino acid metabolism. J Parenter Enteral Nutr 14(4 Suppl): 125S-129S. |
| [6] | Oudemans-van Straaten HM, Bosman RJ, Tresken M, van der Spoel HJ, Zandstra DF. 2001, Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensive Care Med 27(1): 84-90. |
| [7] | Chen QH, Yang Y, He HL, Zie JF, Cai SX, Liu AR, Wang HL, Qiu HB. 2014, The effect of glutamine therapy on outcomes in critically ill patients: a meta-analysis of randomized controlled trials. Critical Care 18:R8 |
| [8] | Bollhalder L, Pfeil AM, Tomonaga Y, Schwenkglenks. 2013, A systematic literature review and meta-analysis of randomized clinical trials of parenteral glutamine supplementation. Clin Nutr 32(2): 213-223. |
| [9] | Pradelli L, Povero M, Muscaritoli M, Eandi M. 2015, Updated cost-effectiveness analysis of supplemental glutamine for parenteral nutrition of intensive-care patients. Eur J Clin Nutr 69: 546-551. |
| [10] | Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, Elke G, Berder MM, Day AG. 2013, A randomized trial of glutamine and antioxidants in critical ill patients. N Engl J Med 368(16): 1489-1497. |
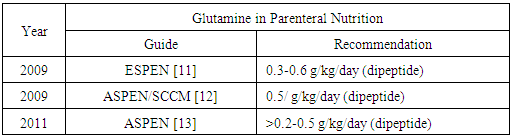
| [11] | Braga M, Ljungqvist O, Soeters P, Fearon K, Weimann A, Bozzetti F. 2009, ESPEN guidelines on parenteral nutrition: surgery. Clin Nutr 28(2009): 378-386. |
| [12] | McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Tylor B, Ochoa JB, Napolitano L, Cresci G, ASPEN Board of Directors, American College of Critical Care Medicine, Society of Critical Care Medicine. 2009, Guidelines for the provision and assessment of nutrition support therapy in the adult critical ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). J Parenter Enteral Nutr 33(3): 277-316. |
| [13] | Vanek VW, Matarese LE, Robinson M, Sacks GS, Kochevar M, Novel Nutrient Task Force, Parenteral Glutamine Workgroup, American Society for Parenteral and Enteral Nutrition (ASPEN), Board Directors. 2011, ASPEN position paper: parenteral nutrition glutamine supplementation. Nutr Clin Pract 26(4): 479-494. |
| [14] | Galvez R, Hirsch S, Klaassen J, Papaprieto V, Reyes E, Ugarte S. 2011, Guías prácticas de soporte nutricional en unidades de cuidado intensivos e intermedio. (Online article), available fromhttp://www.achinumet.cl/guias-soporte/Guias%20Soporte%20Nutricional%20UCI.pdf. Accessed on 14 august 2015. |
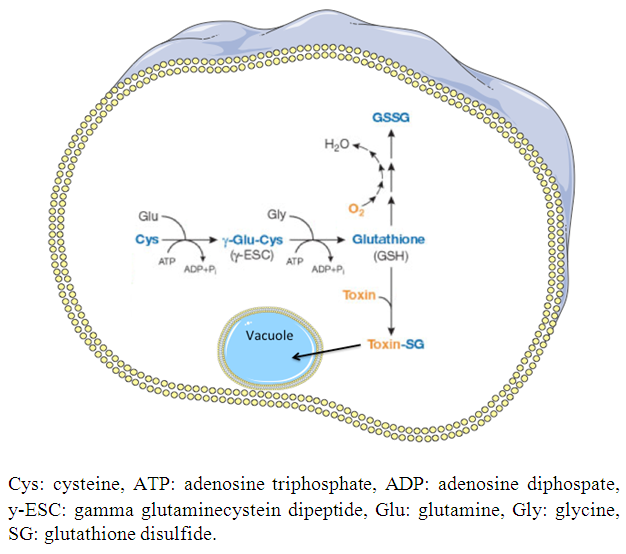
| [15] | Lu SC. 2013, Glutathione synthesis. Biochim Biophys Acta 1830(5): 3143-3153. |
| [16] | Amores-Sánchez MI, Medina MA. 1999, Glutamine as a precursor of glutathione and oxidative stress. Mol Genet Metab 67(2): 100-105. |
| [17] | Wu G, Fang YZ, Yana S, Lupton JR, Turner ND. 2004, Glutathione metabolism and its implications for health. J Nutr 134(3): 489-492. |
| [18] | Liz Z, Srivastava P. 2004, Heat-shock proteins. Curr Protoc Immunol doi: 10.1002/0471142735.ima01ts58. |
| [19] | Feder ME, Hofmann GE. 1999, Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61: 243-282. |
| [20] | Apostolou K, Vardas K, Briassouli E, Psara K, Goukos D, Mageira E, Nanas S, Routsi C, Briassoulis G. 2015, Early heat shock protein 72 and 90 intracellular and extracellular responses in patients with severe sepsis or systemic inflammatory response syndrome. Critical Care 19 (Suppl 1): P41. |
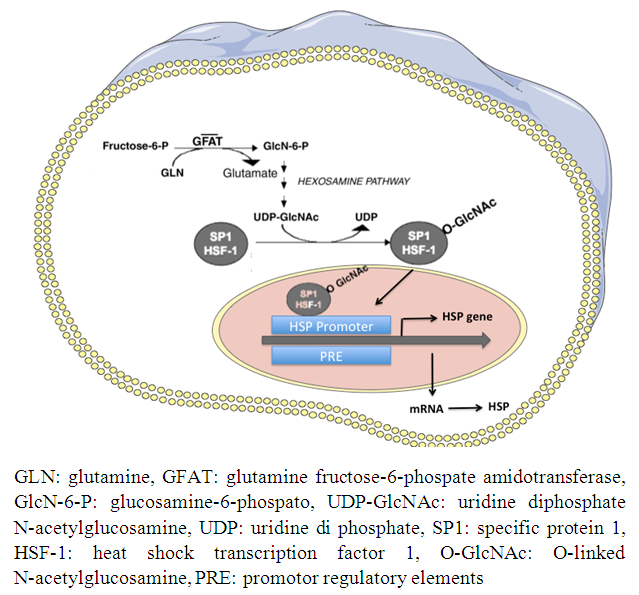
| [21] | Hamiel CR, Pinto S, Hau A, Wischmeyer PE. 2009, Glutamine enhances heat shock protein 70 expression via increased hesoxamine biosynthetic pathway activity. Am J Physiol Cell Physiol 297(6): 1509-1519. |
| [22] | Berg RD. 1999, Bacterial translocation from the gastrointestinal tract. Adv Exp Med Biol 473: 11-30. |
| [23] | McFie J. 2004, Current status of bacterial translocation as a cause of surgical sepsis. Br Med Bull 71(1): 1-11. |
| [24] | Gatt M, Reddy BS, MacFie J. 2007, Review article: bacterial translocation in the critically ill-evidence and methods of prevention. Aliment Pharmacol Ther 25(7): 741-757. |
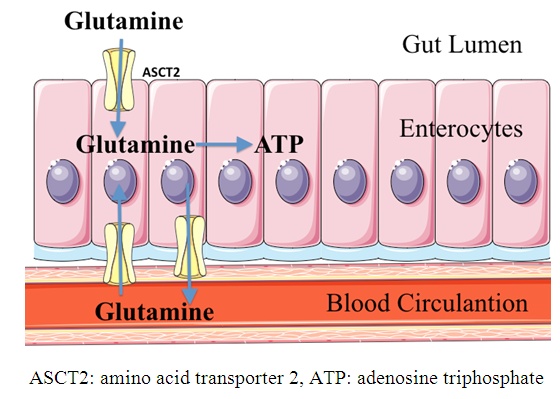
| [25] | McCauley R, Kong SE, Hall J. 1998, Glutamine and nucleotide metabolism within enterocytes. J Parenter Enteral Nutr 22(2): 105-111. |
| [26] | Yang H, Soderholm JD, Larsson J, Pernert J, Lindgreen J, Wirén M. 2000, Bidirectional supply of glutamine maintains enterocyte ATP content in the in vitro using chamber model. Int J Colorectal Dis 15(5-6): 291-296. |
| [27] | Yi D, Hou Y, Wang L, Ouyang W, Long M, Zhao D, Ding B, Liu Y, Wu G. 2015, L-Glutamine enchances enterocyte growth via activation of the mTOR signaling pathway independently of AMPK. Amino Acids 47(1): 65-78. |
| [28] | Pochini L, Scalise M, Galluccio M, Indiveri C. 2014, Membrane transporters fro the special amino glutamine: structure/function relationships and relevance to human health. Front Chem 2:61 doi: 10.3389/fchem.2014.00061. |
| [29] | Clark EC, Patel SD, Chadwick PR, Warhurst G, Curry A, Carlson GL. 2003, Glutamine deprivation facilitates tumor necrosis factor induced bacterial translocation in Caco-2 cells by depletion of enterocytes fuel substrate. Gut 52(2): 224-230. |
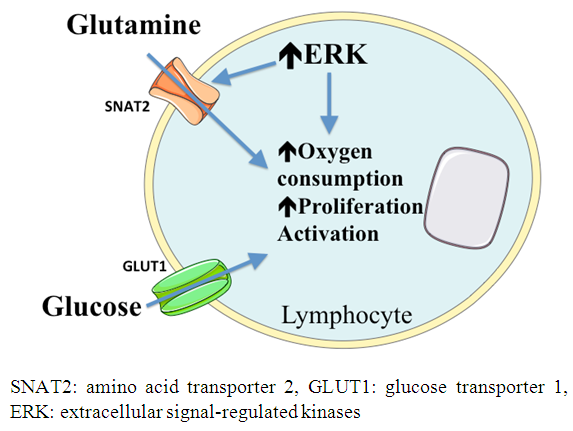
| [30] | Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, Aghvanyan A, Turay AM, Frauwirth KA. 2010, Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK durng T lymphocyte activation.cJ Immunol 185(2): 1037-1044. |
| [31] | Chang WK, Yang KD, Shaio MF. 1999, Lymphocyte proliferation modulated by glutamine: involved the endogenous redox reaction Clin Exp Immunol 117(3): 482-488. |
| [32] | Yaqoob P, Calder PC. 1997, Glutamine requirement of proliferating T lymphocytes. Nutrition 13(7-8): 646-651. |
| [33] | Newsholme P. 2001, Why is L-Glutamine metabolism important to cells of the immune system in health, postinfury, surgery or infection?. J Nutr 131(9): 2515S-2522S. |
| [34] | Wasinkski F, Gregnani MF, Ornellas FH, Bacurau AV, Camara NO, Araujo RC, Bacurau RF. 2014, Lymphocyte flucose and glutamine metabolism as targets of the anti-inflammatory and immunomodulatory effects of exercise. Mediators Inflamm ID 326803. |
| [35] | Estivariz CF, Griffith DP, Luo M, Szeszycki EE, Bazargan N, Dave N, Daignault NM, Bergman GF, McNally T, Battey CH, Furr CE, Hao L, Ramsay JG, Accardi CR, Cotsonis GA, Jones DP, Galloway JR, Ziegler TR. 2008, Efficacy of parental nutrition supplemented with glutamine dipeptide to decrease hospital infections in critical ill surgical patients. J Parenter Enteral Nutr 32(4): 389-402. |
| [36] | Tomé D, Bos Cecile. 2000, Dietary protein and nitrogen utilization. J Nutr, 130(7): 1868S-1873S. |
| [37] | Young VR. 1986, Nutritional balance studies: indicators of human requirements or of adaptive mechanisms?. J Nutr 116: 700-703. |
| [38] | Pitkanen O, Takala J, Poyhonen M, Kari A. 1991, Nitrogen and energy balance in septic and injured intensive care patients: response to parenteral nutrition. Clin Nutr, 10(5): 258-265. |
| [39] | Cheatham ML, Safcsak K, Brzezinski S, Lube M. 2007, Nitrogen balance, protein loss and the open abdomen. Crit Care Med 35(1): 127-131. |
| [40] | Morlion BJ, Stehle P, Wachtler P, Siedhoff HP, Koller M, Konig W, Furst P, Puchstein C. 1998, Total parenteral nutrition with glutamine dipeptide after major abdominal surgery: a randomized, double blind controlled study. Ann Surg, 227(2): 302-308. |
| [41] | Brown MG, Campbell GR, Rowlands BJ. 1991, Glutamine-enhaced enteral diet improves nitrogen balance without increasing portal ammonia. Br J Surg 78(11): 1305-1306. |
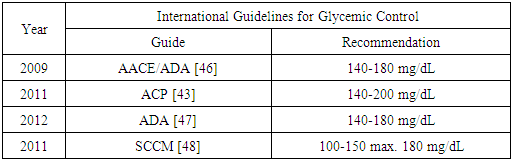
| [42] | Van Ness-Otunnu, Hack JB. 2013, Hyperglycemic crisis. J Emerg Med 45(5): 797-805. |
| [43] | Qassem A, Humphrey LL, Chou R, Snow V, Shekelle PC. 2011, Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: a clinical practice guideline from the American Colleges of Physicians. Ann Intern Med 154(4): 260-267. |
| [44] | Van den Vergue G. 2004, How does blood glucose control with insulin save lives in intensive care?. J Clin Invest 114(9): 1187-1195. |
| [45] | Krinsley JS. 2003, Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc 78(12): 1471-1478. |
| [46] | Moguissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE.2009, American Association of Clinical Endocrinologist and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Cares 32(6): 1119-1131. |
| [47] | American Diabetes Association. 2012, Standards of medical care in diabetes-2012. Diabetes Care 35(suppl1): s11-s63. |
| [48] | Jacobi J, Bircher N, Krinsley J, Agus M, Braithwaite SS, Deutschman, Freire AX, Geehan D, Kohl B, Nasraway SA, Rigby M, Sands K, Schallom L, Taylor B, Umpierrez G, Mazuski J, Schunemann H. 2012, Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med 40(12): 3251-3276. |
| [49] | Molfino A, Logorelli F, Muscaritoli M, Cascino A, Preziosa I, Fanelli FR, Laviano A. 2010, Metabolic effects of glutamine on insulin sensitivity. Nut Therapy Met 28(1): 7-11. |
| [50] | Grintescu IM, Vasiliu IL, Badica IC, Mirea L, Pavelescu D, Balanescu A, Grintescu IC. 2015, The influence of parenteral glutamine supplementation on glucose homeostasis in critically ill polytrauma patients- A randomized-controlled clinical study. Clin Nutr 34: 377-382. |
| [51] | Li C, Buettger C, Kwagh J, Matter A, Daikhin Y, Nissim IB, Collins HW, Yudkoff M, Stanley CA, Matschinsky FM. 2004, A signaling role of glutamine in insulin secretion. J Biol Chem 279(14): 13393-13401. |
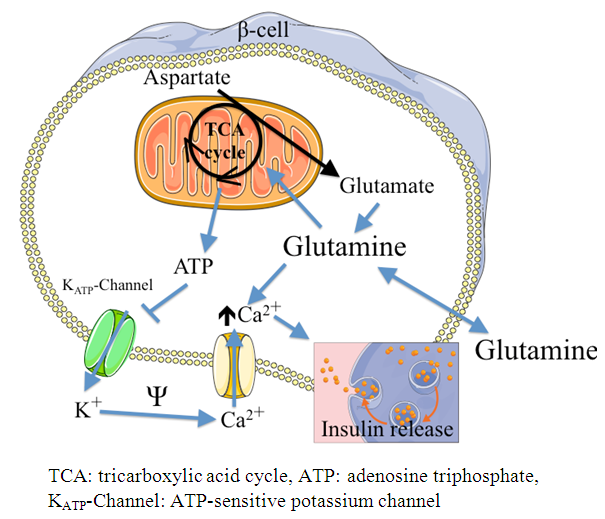
| [52] | Brennan L, Corless M, Hewage C, Malthouse JP, McClenaghan NH, Flatt PR, Newsholme P. 2003, 13C NMR analysis reveals a link between L-glutamine metabolism, D-glucose metabolism and gamma-glutamyl cycle activity in a clonal β pancreatic cell line. Diabetologia 46: 1512-1521. |
| [53] | Perez-Barcena J, Marse P, Zabalegui-Perez A, Corral E, Herrán-Monge M, Cervera M, Llompart-Pou JA, Ayestarán I, Raurich JA, Oliver A, Bruño A, García de Lorenzo A, Frontera G. 2014, A randomized trial of intravenous glutamine supplementation in trauma ICU patients. Intensive Care Med 40:539-547. |
| [54] | Matés JM, Segura JA, Alonso FJ, Márquez J. 2006, Pathways from glutamine to apoptosis. Front Biosci 1(11): 164-180. |
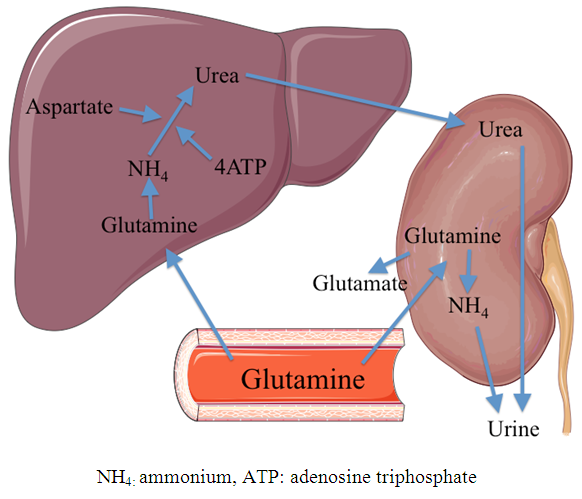
| [55] | Weiner D, Mitch WE, Sands JM. 2014, Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin J Am Soc Nephrol 10(8): 1444-1458. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML