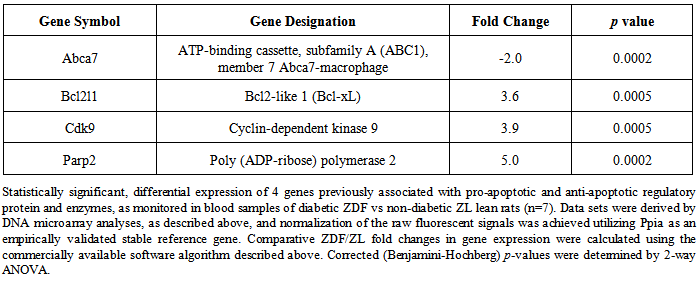
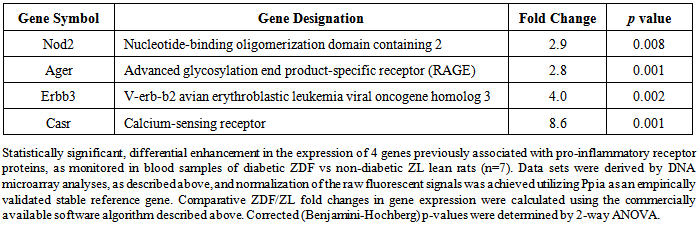
| [1] | Wilson, C.L., Hine, D.W., Pradipta, A., Pearson, J.P., van, E.W., Robinson, J.H., and Knight, A.M., 2012, Presentation of the candidate rheumatoid arthritis autoantigen aggrecan by antigen-specific B cells induces enhanced CD4(+) T helper type 1 subset differentiation, Immunology, 135(4), 344-354. |
| [2] | Cosmi, L., Liotta, F., Maggi, E., Romagnani, S., and Annunziato, F., 2014, Th17 and Non-Classic Th1 Cells in Chronic Inflammatory Disorders: Two Sides of the Same Coin, Int. Arch. Allergy Immunol., 164(3), 171-177. |
| [3] | O'Connell, R.M., Kahn, D., Gibson, W.S., Round, J.L., Scholz, R.L., Chaudhuri, A.A., Kahn, M.E., Rao, D.S., and Baltimore, D., 2010, MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development, Immunity., 33(4), 607-619. |
| [4] | VandeWalle, L., Van Opdenbosch, N., Jacques, P., Fossoul, A., Verheugen, E., Vogel, P., Beyaert, R., Elewaut, D., Kanneganti, T.D., van, L.G., and Lamkanfi, M., 2014, Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis, Nature, 512(7512), 69-73. |
| [5] | Hollan, I., Meroni, P.L., Ahearn, J.M., Cohen Tervaert, J.W., Curran, S., Goodyear, C.S., Hestad, K.A., Kahaleh, B., Riggio, M., Shields, K., and Wasko, M.C., 2013, Cardiovascular disease in autoimmune rheumatic diseases, Autoimmun. Rev., 12(10), 1004-1015. |
| [6] | Ong, K.L., Wu, B.J., Cheung, B.M., Barter, P.J., and Rye, K.A., 2013, Arthritis: its prevalence, risk factors, and association with cardiovascular diseases in the United States, 1999 to 2008,Ann.Epidemiol., 23(2), 80-86. |
| [7] | Carley, A.N. and Severson, D.L., 2005, Fatty acid metabolism is enhanced in type 2 diabetic hearts, Biochim. Biophys. Acta, 1734(2), 112-126. |
| [8] | Donath, M.Y., 2014, Targeting inflammation in the treatment of type 2 diabetes: time to start, Nat. Rev. Drug Discov., 13(6), 465-476. |
| [9] | Lu, M.C., Yan, S.T., Yin, W.Y., Koo, M., and Lai, N.S., 2014, Risk of rheumatoid arthritis in patients with type 2 diabetes: a nationwide population-based case-control study, PLoS. One., 9(7), e101528. |
| [10] | Kakimoto, T., Kimata, H., Iwasaki, S., Fukunari, A., and Utsumi, H., 2013, Automated recognition and quantification of pancreatic islets in Zucker diabetic fatty rats treated with exendin-4, J. Endocrinol., 216(1), 13-20. |
| [11] | Wang, F., Guo, X., Shen, X., Kream, R.M., Mantione, K.J., and Stefano, G.B., 2014, Vascular Dysfunction Associated with Type II Diabetes and Alzheimer's Disease: A Potential Etiological Linkage, Med SciMonit Basic Res, 20, 118-129. |
| [12] | Zanchi, C., Locatelli, M., Benigni, A., Corna, D., Tomasoni, S., Rottoli, D., Gaspari, F., Remuzzi, G., and Zoja, C., 2013, Renal expression of FGF23 in progressive renal disease of diabetes and the effect of ACE inhibitor, PLoS. One., 8(8), e70775. |
| [13] | Mierzecki, A., Kloda, K., Bukowska, H., Chelstowski, K., Makarewicz-Wujec, M., and Kozlowska-Wojciechowska, M., 2013, Association between low-dose folic acid supplementation and blood lipids concentrations in male and female subjects with atherosclerosis risk factors, Med SciMonit, 19, 733-739. |
| [14] | Stohr, R. and Federici, M., 2013, Insulin resistance and atherosclerosis: convergence between metabolic pathways and inflammatory nodes, Biochem.J., 454(1), 1-11. |
| [15] | Spalenza, V., Girolami, F., Bevilacqua, C., Riondato, F., Rasero, R., Nebbia, C., Sacchi, P., and Martin, P., 2011, Identification of internal control genes for quantitative expression analysis by real-time PCR in bovine peripheral lymphocytes, Vet. J., 189(3), 278-283. |
| [16] | Gholam, P.M., Flancbaum, L., Machan, J.T., Charney, D.A., and Kotler, D.P., 2007, Nonalcoholic fatty liver disease in severely obese subjects, Am. J. Gastroenterol., 102(2), 399-408. |
| [17] | Tiniakos, D.G., Vos, M.B., and Brunt, E.M., 2010, Nonalcoholic fatty liver disease: pathology and pathogenesis, Annu. Rev. Pathol., 5, 145-171. |
| [18] | Cho, Y.E., Basu, A., Dai, A., Heldak, M., and Makino, A., 2013, Coronary endothelial dysfunction and mitochondrial reactive oxygen species in type 2 diabetic mice, Am. J. Physiol Cell Physiol, 305(10), C1033-C1040. |
| [19] | Welters, I.D., Fimiani, C., Bilfinger, T.V., and Stefano, G.B., 1999, NF-kB, nitric oxide and opiate signaling, Medical Hypotheses, 54(2), 263-268. |
| [20] | Majdalawieh, A. and Ro, H.S., 2010, Regulation of Ikappa Balpha function and NF-kappaB signaling: AEBP1 is a novel proinflammatory mediator in macrophages, Mediators. Inflamm., 2010, 823821. |
| [21] | Shen, J., Yang, M., Ju, D., Jiang, H., Zheng, J.P., Xu, Z., and Li, L., 2010, Disruption of SM22 promotes inflammation after artery injury via nuclear factor kappaB activation, Circ.Res, 106(8), 1351-1362. |
| [22] | Ikeda, D., Ageta, H., Tsuchida, K., and Yamada, H., 2013, iTRAQ-based proteomics reveals novel biomarkers of osteoarthritis, Biomarkers, 18(7), 565-572. |
| [23] | Yoon, H.Y., Lee, E.G., Lee, H., Cho, I.J., Choi, Y.J., Sung, M.S., Yoo, H.G., and Yoo, W.H., 2013, Kaempferol inhibits IL-1beta-induced proliferation of rheumatoid arthritis synovial fibroblasts and the production of COX-2, PGE2 and MMPs, Int. J. Mol. Med, 32(4), 971-977. |
| [24] | Yun, H.M., Park, K.R., Lee, H.P., Lee, D.H., Jo, M., Shin, D.H., Yoon, D.Y., Han, S.B., and Hong, J.T., 2014, PRDX6 promotes lung tumor progression via its GPx and iPLA2 activities, Free Radic. Biol. Med, 69, 367-376. |
| [25] | Luiking, Y.C., Ten Have, G.A., Wolfe, R.R., and Deutz, N.E., 2012, Arginine de novo and nitric oxide production in disease states, Am. J. Physiol Endocrinol. Metab, 303(10), E1177-E1189. |
| [26] | Beier, U.H., Wang, L., Han, R., Akimova, T., Liu, Y., and Hancock, W.W., 2012, Histone deacetylases 6 and 9 and sirtuin-1 control Foxp3+ regulatory T cell function through shared and isoform-specific mechanisms, Sci Signal., 5(229), ra45. |
| [27] | Tao, R., de Zoeten, E.F., Ozkaynak, E., Chen, C., Wang, L., Porrett, P.M., Li, B., Turka, L.A., Olson, E.N., Greene, M.I., Wells, A.D., and Hancock, W.W., 2007, Deacetylase inhibition promotes the generation and function of regulatory T cells, Nat. Med, 13(11), 1299-1307. |
| [28] | Hua, K.F., Wang, S.H., Dong, W.C., Lin, C.Y., Ho, C.L., and Wu, T.H., 2012, High glucose increases nitric oxide generation in lipopolysaccharide-activated macrophages by enhancing activity of protein kinase C-alpha/delta and NF-kappaB, Inflamm. Res, 61(10), 1107-1116. |
| [29] | Jancinova, V., Perecko, T., Nosal, R., Kostalova, D., Bauerova, K., and Drabikova, K., 2009, Decreased activity of neutrophils in the presence of diferuloylmethane (curcumin) involves protein kinase C inhibition, Eur. J. Pharmacol., 612(1-3), 161-166. |
| [30] | Liu, C.J., 2011, Progranulin: a promising therapeutic target for rheumatoid arthritis, FEBS Lett., 585(23), 3675-3680. |
| [31] | Tang, W., Lu, Y., Tian, Q.Y., Zhang, Y., Guo, F.J., Liu, G.Y., Syed, N.M., Lai, Y., Lin, E.A., Kong, L., Su, J., Yin, F., Ding, A.H., Zanin-Zhorov, A., Dustin, M.L., Tao, J., Craft, J., Yin, Z., Feng, J.Q., Abramson, S.B., Yu, X.P., and Liu, C.J., 2011, The growth factor progranulin binds to TNF receptors and is therapeutic against inflammatory arthritis in mice, Science, 332(6028), 478-484. |
| [32] | Thurner, L., Preuss, K.D., Fadle, N., Regitz, E., Klemm, P., Zaks, M., Kemele, M., Hasenfus, A., Csernok, E., Gross, W.L., Pasquali, J.L., Martin, T., Bohle, R.M., and Pfreundschuh, M., 2013, Progranulin antibodies in autoimmune diseases, J. Autoimmun., 42, 29-38. |
| [33] | Deng, H., Maitra, U., Morris, M., and Li, L., 2013, Molecular mechanism responsible for the priming of macrophage activation, J. Biol. Chem., 288(6), 3897-3906. |
| [34] | Chang, L.H., Huang, H.S., Wu, P.T., Jou, I.M., Pan, M.H., Chang, W.C., Wang, D.D., and Wang, J.M., 2012, Role of macrophage CCAAT/enhancer binding protein delta in the pathogenesis of rheumatoid arthritis in collagen-induced arthritic mice, PLoS. One., 7(9), e45378. |
| [35] | Duvallet, E., Semerano, L., Assier, E., Falgarone, G., and Boissier, M.C., 2011, Interleukin-23: a key cytokine in inflammatory diseases, Ann. Med, 43(7), 503-511. |
| [36] | Jawien, J., Toton-Zuranska, J., Kus, K., Pawlowska, M., Olszanecki, R., and Korbut, R., 2012, The effect of AVE 0991, nebivolol and doxycycline on inflammatory mediators in an apoE-knockout mouse model of atherosclerosis, Med SciMonit, 18(10), BR389-BR393. |
| [37] | Jenkins, E., Brenner, M., Laragione, T., and Gulko, P.S., 2012, Synovial expression of Th17-related and cancer-associated genes is regulated by the arthritis severity locus Cia10, Genes Immun., 13(3), 221-231. |
| [38] | Rosengren, S., Corr, M., and Boyle, D.L., 2010, Platelet-derived growth factor and transforming growth factor beta synergistically potentiate inflammatory mediator synthesis by fibroblast-like synoviocytes, Arthritis Res Ther., 12(2), R65. |
| [39] | Radons, J., Gabler, S., Wesche, H., Korherr, C., Hofmeister, R., and Falk, W., 2002, Identification of essential regions in the cytoplasmic tail of interleukin-1 receptor accessory protein critical for interleukin-1 signaling, J. Biol. Chem., 277(19), 16456-16463. |
| [40] | Lee, J.C., Espeli, M., Anderson, C.A., Linterman, M.A., Pocock, J.M., Williams, N.J., Roberts, R., Viatte, S., Fu, B., Peshu, N., Hien, T.T., Phu, N.H., Wesley, E., Edwards, C., Ahmad, T., Mansfield, J.C., Gearry, R., Dunstan, S., Williams, T.N., Barton, A., Vinuesa, C.G., Parkes, M., Lyons, P.A., and Smith, K.G., 2013, Human SNP links differential outcomes in inflammatory and infectious disease to a FOXO3-regulated pathway, Cell, 155(1), 57-69. |
| [41] | Lutzner, N., Kalbacher, H., Krones-Herzig, A., and Rosl, F., 2012, FOXO3 is a glucocorticoid receptor target and regulates LKB1 and its own expression based on cellular AMP levels via a positive autoregulatory loop, PLoS.One., 7(7), e42166. |
| [42] | Berrebi, D., Bruscoli, S., Cohen, N., Foussat, A., Migliorati, G., Bouchet-Delbos, L., Maillot, M.C., Portier, A., Couderc, J., Galanaud, P., Peuchmaur, M., Riccardi, C., and Emilie, D., 2003, Synthesis of glucocorticoid-induced leucine zipper (GILZ) by macrophages: an anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and IL-10, Blood, 101(2), 729-738. |
| [43] | Cheng, Q., Morand, E., and Yang, Y.H., 2014, Development of novel treatment strategies for inflammatory diseases-similarities and divergence between glucocorticoids and GILZ, Front Pharmacol., 5, 169. |
| [44] | Cheng, Q., Fan, H., Ngo, D., Beaulieu, E., Leung, P., Lo, C.Y., Burgess, R., van der Zwan, Y.G., White, S.J., Khachigian, L.M., Hickey, M.J., and Morand, E.F., 2013, GILZ overexpression inhibits endothelial cell adhesive function through regulation of NF-kappaB and MAPK activity, J. Immunol., 191(1), 424-433. |
| [45] | Chapman, K.E., Coutinho, A.E., Zhang, Z., Kipari, T., Savill, J.S., and Seckl, J.R., 2013, Changing glucocorticoid action: 11beta-hydroxysteroid dehydrogenase type 1 in acute and chronic inflammation, J. Steroid Biochem. Mol. Biol., 137, 82-92. |
| [46] | Moon, S.S., Lee, Y.S., Kim, J.G., Kim, S.W., Jeong, J.Y., Jeon, E.J., Seo, H.A., Kwak, S.H., Park, K.S., and Lee, I.K., 2011, Relationship of 11beta-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase gene polymorphisms with metabolic syndrome and type 2 diabetes, Endocr.J., 58(11), 949-959. |
| [47] | Jehle, A.W., Gardai, S.J., Li, S., Linsel-Nitschke, P., Morimoto, K., Janssen, W.J., Vandivier, R.W., Wang, N., Greenberg, S., Dale, B.M., Qin, C., Henson, P.M., and Tall, A.R., 2006, ATP-binding cassette transporter A7 enhances phagocytosis of apoptotic cells and associated ERK signaling in macrophages, J. Cell Biol., 174(4), 547-556. |
| [48] | Wu, B.J., Ong, K.L., Shrestha, S., Chen, K., Tabet, F., Barter, P.J., and Rye, K.A., 2014, Inhibition of arthritis in the Lewis rat by apolipoprotein A-I and reconstituted high-density lipoproteins, Arterioscler. Thromb. Vasc. Biol., 34(3), 543-551. |
| [49] | Sawatzky, D.A., Willoughby, D.A., Colville-Nash, P.R., and Rossi, A.G., 2006, The involvement of the apoptosis-modulating proteins ERK 1/2, Bcl-xL and Bax in the resolution of acute inflammation in vivo, Am.J.Pathol., 168(1), 33-41. |
| [50] | Bardwell, P.D., Gu, J., McCarthy, D., Wallace, C., Bryant, S., Goess, C., Mathieu, S., Grinnell, C., Erickson, J., Rosenberg, S.H., Schwartz, A.J., Hugunin, M., Tarcsa, E., Elmore, S.W., McRae, B., Murtaza, A., Wang, L.C., and Ghayur, T., 2009, The Bcl-2 family antagonist ABT-737 significantly inhibits multiple animal models of autoimmunity, J.Immunol., 182(12), 7482-7489. |
| [51] | Rossi, A.G., Sawatzky, D.A., Walker, A., Ward, C., Sheldrake, T.A., Riley, N.A., Caldicott, A., Martinez-Losa, M., Walker, T.R., Duffin, R., Gray, M., Crescenzi, E., Martin, M.C., Brady, H.J., Savill, J.S., Dransfield, I., and Haslett, C., 2006, Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis, Nat.Med, 12(9), 1056-1064. |
| [52] | Schmerwitz, U.K., Sass, G., Khandoga, A.G., Joore, J., Mayer, B.A., Berberich, N., Totzke, F., Krombach, F., Tiegs, G., Zahler, S., Vollmar, A.M., and Furst, R., 2011, Flavopiridol protects against inflammation by attenuating leukocyte-endothelial interaction via inhibition of cyclin-dependent kinase 9,Arterioscler.Thromb.Vasc.Biol., 31(2), 280-288. |
| [53] | Wang, K., Hampson, P., Hazeldine, J., Krystof, V., Strnad, M., Pechan, P., and J, M., 2012, Cyclin-dependent kinase 9 activity regulates neutrophil spontaneous apoptosis, PLoS. One., 7(1), e30128. |
| [54] | Peralta-Leal, A., Rodriguez-Vargas, J.M., Aguilar-Quesada, R., Rodriguez, M.I., Linares, J.L., de Almodovar, M.R., and Oliver, F.J., 2009, PARP inhibitors: new partners in the therapy of cancer and inflammatory diseases, Free Radic. Biol. Med, 47(1), 13-26. |
| [55] | Chen, Y., Sun, W., Gao, R., Su, Y., Umehara, H., Dong, L., and Gong, F., 2013, The role of high mobility group box chromosomal protein 1 in rheumatoid arthritis, Rheumatology.(Oxford), 52(10), 1739-1747. |
| [56] | Koulis, C., Kanellakis, P., Pickering, R.J., Tsorotes, D., Murphy, A.J., Gray, S.P., Thomas, M.C., Jandeleit-Dahm, K.A., Cooper, M.E., and Allen, T.J., 2014, Role of bone-marrow- and non-bone-marrow-derived receptor for advanced glycation end-products (RAGE) in a mouse model of diabetes-associated atherosclerosis, Clin.Sci (Lond), 127(7), 485-497. |
| [57] | Correa, R.G., Milutinovic, S., and Reed, J.C., 2012, Roles of NOD1 (NLRC1) and NOD2 (NLRC2) in innate immunity and inflammatory diseases, Biosci. Rep., 32(6), 597-608. |
| [58] | Ospelt, C., Brentano, F., Jungel, A., Rengel, Y., Kolling, C., Michel, B.A., Gay, R.E., and Gay, S., 2009, Expression, regulation, and signaling of the pattern-recognition receptor nucleotide-binding oligomerization domain 2 in rheumatoid arthritis synovial fibroblasts, Arthritis Rheum., 60(2), 355-363. |
| [59] | Yao, Q., 2013, Nucleotide-binding oligomerization domain containing 2: structure, function, and diseases, Semin. Arthritis Rheum., 43(1), 125-130. |
| [60] | Gompels, L.L., Malik, N.M., Madden, L., Jin, P., Feldmann, M., Shepard, H.M., and Paleolog, E.M., 2011, Human epidermal growth factor receptor bispecific ligand trap RB200: abrogation of collagen-induced arthritis in combination with tumour necrosis factor blockade, Arthritis Res Ther., 13(5), R161. |
| [61] | Swanson, C.D., Akama-Garren, E.H., Stein, E.A., Petralia, J.D., Ruiz, P.J., Edalati, A., Lindstrom, T.M., and Robinson, W.H., 2012, Inhibition of epidermal growth factor receptor tyrosine kinase ameliorates collagen-induced arthritis, J. Immunol., 188(7), 3513-3521. |
| [62] | Malecki, R., Fiodorenko-Dumas, Z., Jakobsche-Policht, U., Malodobra, M., and Adamiec, R., 2013, Altered monocyte calcium-sensing receptor expression in patients with type 2 diabetes mellitus and atherosclerosis, J. Physiol Pharmacol., 64(4), 521-527. |
| [63] | Paccou, J., Boudot, C., Renard, C., Liabeuf, S., Kamel, S., Fardellone, P., Massy, Z., Brazier, M., and Mentaverri, R., 2014, Total calcium-sensing receptor expression in circulating monocytes is increased in rheumatoid arthritis patients with severe coronary artery calcification, Arthritis Res Ther., 16(5), 412. |

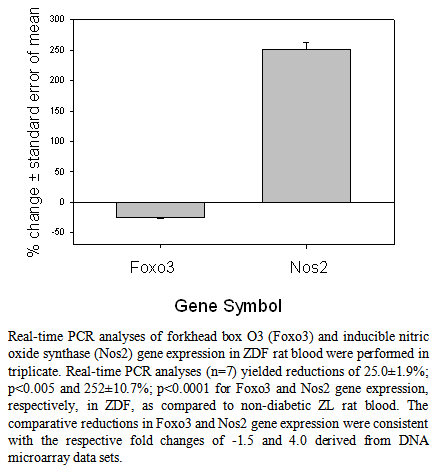
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML