-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Psychology and Behavioral Sciences
p-ISSN: 2163-1948 e-ISSN: 2163-1956
2020; 10(3): 51-62
doi:10.5923/j.ijpbs.20201003.01

Obsessions and Musical Hallucinations: The Neurobiology Underlying a Differential Diagnosis
Mayra E. Dávila-Rivera, Hilda M. Rivera-Marín
Albizu University, San Juan Campus, San Juan, Puerto Rico
Correspondence to: Hilda M. Rivera-Marín, Albizu University, San Juan Campus, San Juan, Puerto Rico.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This literature review has the purpose of establishing the difference between comorbid disorders with musical obsessions and hallucinations from a neurobiological perspective. Musical obsessions and hallucinations are unusual, with limited research, and their prevalence is underestimated [1,2]. Their pathological etiology can be confusing since it can fall within psychiatry, neurology and biology due to its symptomatic comorbidity; and it may not be recognized as generating suffering for the patient [1]. Neurobiology has made it possible to increase our knowledge of brain structures or circuits responsible for the behavior of subjects who suffer related disorders. Musical obsessions and hallucinations are disabling as they are detrimental to quality of life. Due to new discoveries in the different scientific disciplines, health professionals will establish a differential diagnosis, adequately address primary and underlying pathologies, and also facilitate the implementation of effective evidence-based models.
Keywords: Disorders, Musical hallucinations, Musical obsessions, Neurobiology
Cite this paper: Mayra E. Dávila-Rivera, Hilda M. Rivera-Marín, Obsessions and Musical Hallucinations: The Neurobiology Underlying a Differential Diagnosis, International Journal of Psychology and Behavioral Sciences, Vol. 10 No. 3, 2020, pp. 51-62. doi: 10.5923/j.ijpbs.20201003.01.
Article Outline
1. Introduction
- Research has identified various disorders sharing similar characteristics within the neurobiological model of the prefrontal cortex. The comorbidity that exists between psychiatric, neurological and diseases associated with hearing can confuse the cause of musical obsessions and hallucinations. Diverse authors define musical obsessions or “stuck song syndrome”, as an internal and intrusive repetition of stereotyped musical fragments [2,3,4]. For their part, musical hallucinations are usually in the head. They could have their origin in external stimuli and are perceived with a great sense of reality [5,6]. They are characterized by the sudden appearance of songs or melodies familiar to the patient, which are usually vocals with instrumental accompaniment and religious content [7,8]. Since musical obsessions and hallucinations are considered a pathology between psychiatry, neurology and otorhinolaryngology, they may not be recognized and generate great suffering to the patient [1]. The clinical researchers have underestimated the prevalence and the importance of musical obsessions [2]. Musical obsessions and hallucinations are an unusual phenomenon [1]. Because they are uncommon, they can be classified as a rare disease, which are unattractive as a clinical research focus [9]. These conditions are chronic and disabling characterized by their low prevalence (less than 1,200 individuals), and high morbidity. They are scarcely researched, with more than 80% originating genetically; many of them without a specific treatment. For 65% of the people affected by them, it represents a serious or debilitating disease [5].Advances in neuroimaging, neuroanatomical, and neuropsychological research conducted in the field of mental health have given rise to neurobiological theories and models. These explain the relationship of behavior with different alterations in brain structures or circuits. Neurobiology has provided a greater understanding of the etiology of these pathologies, by studying the functioning of the nervous system, its organization, and being an essential foundation of behavioral, cognitive and emotional behavior [10]. Neurobiology is useful for both mental health professionals and patients to understand the course of the disorder, the management of interventions, and the treatment plan. As knowledge increases, the hope is to develop and validate predictive algorithms to optimize diagnosis, management, and treatment at the individual patient level [11]. Cárdenas-Rodríguez et al. (2019) and Casey et al. (2009), described impulsive behavior within the context of the neurobiological model of the prefrontal cortex and its connectivity with temporal limbic structures (hippocampus, amygdala and hypothalamus). Impulsiveness is a predisposition to a behavior that involves quick, unplanned actions, that urge immediate reinforcement [12,13,14]. Evidence has been found that there is a connectivity between the nucleus accumbens and orbitofrontal cortex that allows the regulation of impulsive behavior. Knowing the symptomatic differences between musical obsessions and hallucinations makes it easier for the health professional to describe, understand, diagnose and better manage the symptoms that are disabling for many patients [5]. It is essential to establish an accurate diagnosis to implement adequate treatments that reduce the patient’s anxiety, who sometimes do not understand where the endless music in their head comes from [1].
2. Methodology
- The main objective of this literature review is to offer an updated descriptive framework of the most relevant empiric evidence of the different mental, neurological and organic disorders from a neurobiological perspective. The purpose is to establish the differences between musical obsessions and hallucinations, despite the symptomatic overlaps between their diagnoses. The unusual knowledge of musical obsessions and hallucinations present in different neurobiological disorders will be disseminated to create awareness of them. In addition, it may encourage health professionals to thoroughly explore the presence of symptoms related to musical obsessions or hallucinations, or both. The review consists of an extensive bibliographic scrutiny of research published from 1971 to March 2020. The research was grouped into three main areas of study: musical obsessions and hallucinations, comorbidity between diagnoses and neurobiology of the disorders involved.
3. Discussion
3.1. Musical Imagination and Earworms
- Orjuela-Rojas & Lizarazo-Rodríguez (2018) established the difference between these phenomena. They explain that almost all human beings can remember music voluntarily. Musical imagination is more related to the artistic environment [2]. Music is universal, has inherently memorable qualities and can become catchy [4]. Sticky songs known as “earworms”, are fragments of music that arise spontaneously, constantly repeating themselves in the mind, causing distraction. However, the active discomfort experienced could be temporarily, lasting for minutes or hours. It is estimated that 98% of Western population experience them, but they do not reach a clinical level of severity, such as musical obsession [2,3,4]. In a clinical case presented by Orjuela-Rojas & Lizarazo-Rodríguez (2018), the evolution from earworm to musical obsession was evident, due to the lack of control and severity of symptoms [2]. A 32-year old woman, with significant anxiety problems, reported symptoms of distress and insomnia from repeatedly listening to musical fragments in her head for a period of 12 years. Initially intermittent, and then recurring, she listened to “jingles” from television commercials, instrument fragments, and vocal pieces. Over time, her concentration, interpersonal relationships and academic performance deteriorated. At the same time, Rafin (2016) includes a clinical case of a 19-year old university student with constant music complaints in his head for the past three years. Over time, he began to worsen, reporting experiencing intrusive sexual and violent images, which coincided with academic burden, social isolation, and introverted personality [4]. These two cases are consistent with others published in the literature, in which almost all patients with musical obsessions are diagnosed with Obsessive Compulsive Disorder (OCD) [4,15].
3.2. Distinction between Musical Obsessions and Hallucinations
- Musical obsessions and hallucinations are confused with each other. They can arise in various psychiatric and neurological pathologies such as schizophrenia, OCD, personality disorders, mood, anxiety, motor, tumors, epilepsy, problematic substance use, some types of neurodegenerative and neurodevelopmental conditions, among others [1,5]. In general, musical obsessions are fragments of music that the person has heard in the past. They can be meaningful or sometimes even ringtones. Regarding their duration, symptomatic episodes can vary from months to years with continuous or intermittent intensity [2]. Alternatively, cognitive theory considers that obsessions are the result of dysfunctional beliefs related to perfectionism, intolerance to uncertainty, extreme need to control thoughts, inflated responsibility and overestimation of a threat [4,16,17].In contrast, musical hallucinations have not been heard by the patient since childhood or youth. They are usually annoying and irritable, provoke intense anguish over their recurrence, and are experienced with greater intensity in silence [1]. Research indicates that, in most cases, the chants arise at any time of the day, are persistent and disappear when the subject starts another activity or listens to real music [8,18]. Musical hallucinations do not necessarily affect people with a pathological disorder. Other underlying causes have been found, such as serious hearing problems, although in some cases their origin is unknown [1]. Mortiz & Laroi (2008) carried out a survey with 160 subjects, of whom 45 have schizophrenia, 55 with OCD and 60 in the control group. The findings reflected that 7% of the control group reported having heard voices [19]. Furthermore, hallucinations are classified as either functional or organic. The functional ones correspond to psychopathological disorders where there is no presence of brain damage. The organic ones, for the most part, occur in patients with severe acquired hearing loss, such as Charles Bonnet’s auditory syndrome (acquired deafness) or for other causes as injuries, brain tumors, drugs, temporary epilepsy, among others [1,20,21,22,23].Some authors, such as Gómez-Esteban & Robles de la Puente (2015), Rangell (2011), and Sacks (2009), consider music to be a reflection of moods, and link hallucinations with experience and personality [8,24,25]. Despite the fact that few cases of musical hallucinations have been documented, both in children and adults, both share similar clinical characteristics, such as a lack of knowledge and etiology. In relation to the child population, children from the age of three show the ability to recognize happiness in music. Like adults, they can identify sadness, fear, and anger in music starting at age 6 [26,27]. Moreover, clinical cases of musicogenic epilepsy have been reported. The patients are triggered by the emotional content when remembering or thinking about music, some styles of music such as jazz, classical and pop, or instrumental [26].Concerning diagnosis, Gómez-Esteban & Robles de la Puente (2015) found that there are no differences regarding musical intrusions that arise in a similar way, both in neurosis and psychosis. Patients diagnosed with the spectrum of psychosis interpret these according to their level of consciousness about the disease. However, in the obsessive spectrum musical obsessions are interpreted as part of the disease, due to the sudden way in which they manifest [8].To establish a differential diagnosis between musical imagination, “earworms”, obsessions and musical hallucinations, the researchers have proposed different criteria. Kneisel (2018) and Orjuela-Rojas & Lizarazo-Rodríguez (2018) proposed that earworms be classified as a musical obsession due to the person’s inability to end the musical repetition or attempt to distract from it even in the presence of another musical stimulus [2,3]. Similarly, Saba & Keshavan (1997) suggested that the criterion should be the lack of voluntary control [28]. Others differentiate the interpretation of the phenomenon by psychosis for music hallucinations and obsessive neurosis for musical obsessions [1,6,29]. Meanwhile, others indicate that there is a need for consistency in its clinical definitions. They recommend using the term “musical obsessions” to refer to the intrusive and inappropriate thoughts that patients with OCD experience. In contrast to musical hallucinations, which are auditory hallucinations of musical content that can be presented by patients with hearing loss, cognitive impairment, schizophrenia, among others [30,31]. Finally, there are authors who have established the importance of understanding the history of trauma when working with patients who experience OCD in order to provide an adequate diagnosis [32].
3.3. Prevalence of Musical Obsessions /Hallucinations and Obsessive-Compulsive Disorder (TOC)
- The prevalence of musical obsessions is not clearly established, with few cases reported in the world [2]. Nevertheless, there is a demographic pattern in young adults 33 years of age, with no gender difference [2,15]. Earworms are rare and go unnoticed by various diagnostic processes [3]. Only 100 cases have been reported worldwide and it is more common in young adults in their mid 30’s [3,15]. Likewise, the prevalence of musical hallucinations is not well established in the neurological literature and is an underdiagnosed and unusual pathology [19]. However, musical hallucinations affect 2%; probably being the most frequent type of non-psychotic hallucinations [18]. In addition, Warner & Ariz (2005) and Zabalza-Estévez (2014) reported that annually there is one case of musical hallucinations for every 100,000 habitants with psychiatric pathology over 65 years of age [1,33].Musical auditory hallucinations in the psychiatric population are rarer than auditory verbal hallucinations [34]. In the study by Fukunishi et al. (1998) with 3,678 patients, the prevalence averages 0.16%. [35]. Another study by Hermesh et al. (2004) indicated that lifetime prevalence of musical hallucinations of 20% in a population of psychiatric outpatients; in OCD, a rate of 40% was found [36]. In addition, in 132 other cases published up to 2004, the psychiatric origin ranked second. These were divided into five groups according to their etiology: hearing loss, epilepsy, focal lesions, psychiatric disorders, and syndromes related to problematic substance use. The prevalence was higher in women (70%), with a mean age of 61.5 years [34,37,38]. In young patients, they found that brain injuries were the most frequent cause [8].OCD is one of the most investigated, debilitating, widespread, and costly disorders that a person can experience [32,39]. In OCD, obsessions, intrusive and uncontrolled compulsions have a prevalence of 2% [40,41]. The lifetime prevalence of OCD is between 1% to 3%, and patients can experience chronic or episodic OCD throughout their lives [42]. Fenske & Petersen (2015) explained that lifetime prevalence may be unrepresentative, since patients with moderate and severe symptoms are those who seek help. OCD has a bimodal incidence with spikes during late childhood/early adolescence, and again in early adulthood [43]. The prevalence of childhood OCD is 1% to 2% in the United States, and 50% of those have comorbidity with psychiatric conditions [39]. The average age for onset is 19.5 years and rarely develops before 30 years of age [43]. OCD is a chronic disorder and in 60% to 70% of cases it can persist, if it is not identified and treated effectively. [42,43,44,45]. In terms of gender, Blair & Lutz et al. (2012) and Gómez-Esteban & Robles de la Puente (2015) found that obsessions are more prevalent in women than men [8,39]. The lifetime risk of OCD development is higher in women, and usually arises during adolescence [43]. On the other hand, other authors have reported that OCD symptoms tend to worsen in some women during their premenstrual period, linked to hormonal changes. The obsessions are usually related to religious and sexual themes, accompanied by depression, anxiety and suicidal thoughts [41,46]. Fenske & Petersen (2015) indicated that women who are pregnant, or in the postpartum period, show a greater tendency (1.5 to 2 times) to experience OCD than the general population of women [43]. The most relevant intrusive thoughts in women diagnosed with OCD during postpartum were related to harming the infant; however, they do not represent a greater risk of hurting them. Finally, women who have a primary diagnosis of OCD, prior to pregnancy, are more vulnerable to postpartum depression [43,47,48].
3.4. Comorbidity between Disorders: Relationship with Musical Obsessions and Hallucinations
- The term comorbidity or associated comorbidity is used to describe the presence of two or more disorders or diseases that occur simultaneously in the same person. It also implies that both disorders can share characteristics, symptoms that interact with each other; aggravating the evolution and prognosis of both [49,50]. As a result, the patient could experience the sum of multiple symptoms [51]. The mere presence of a disorder comorbid with OCD generates greater personal suffering, detrimentally impacting therapeutic management [52].Neurobiological investigations have shown comorbidity of OCD with other disorders, such as anxiety, post-traumatic stress, spectrum of schizophrenia and other psychotic disorders, neurodevelopmental disorders, motor disorders, and body dysmorphic disorder, among others [8,31,34,53].
3.4.1. OCD: Musical Obsessions and Hallucinations
- OCD is characterized by the presence of obsessions, compulsions, or both. Obsessions are manifested by unwanted intrusive thoughts, images or recurring and persistent impulses (violent and sexual fantasies, among others), causing significant anxiety and distress. There is a struggle to ignore, suppress, or neutralize these intrusive thoughts with another thought or act through compulsive behavior. Compulsions are ritual acts (washing hands, checking things, security, avoiding hurting loved ones, avoiding places, events or people, organization and symmetry, praying, counting, repeating, repetitive questions, seeking approval, difficulty making decisions, and procrastination, among others), which are part of the coping strategies used by the patient [17,39,40,43,54,55].OCD obsessions and compulsions are disabling, causing dysfunction in the patient due to hyperactivity in some brain areas. Symptoms such as cognitive rigidity, increased worry and feelings of guilt are manifested [41]. The World Health Organization has ranked OCD as one of the top 20 causes of disability for individuals age 15-44 [39]. According to a mental health study in the United States, 90% of adults who reported having OCD also had at least one other comorbid condition such as anxiety, mood disorder, attention hyperactivity disorder, oppositional defiant disorder and substance abuse disorder [39]. In another research of 137 patients, 72% experienced perpetual intrusions such as recreating personal obsessions [56]. From studies with twins, it has been established that genetics contribute to risk, with 45% to 65% developing the disorder [39]. Research has shown that at least one third of people with OCD may suffer from musical obsessions, which are more common in this disorder than in other psychiatric disorders [5,29].
3.4.2. OCD: Musical Obsessions and Hallucinations in Post-Traumatic Stress Disorder (PTSD)
- Research on patients with comorbidity of OCD and PTSD indicates that the probability of presenting one of the two disorders is between three and ten times higher, if one of them has been previously diagnosed [57,58,59]. Multiple retrospective studies with samples from obsessive patients indicate a prevalence with PTSD of between 6% and 25% [58,60,61]. Scientific evidence sustains the impact of trauma on OCD is unquestionable, complicating its treatment and prognosis [32,60,62]. Dyskshoorn (2014) and Gershuny & Thayer (1999) suggested that there is an overlap between the symptoms of OCD and PTSD, as both are characterized by intrusive and recurring thoughts, which include fear and anxiety. These authors found that a reduction in PTSD symptoms increases those of OCD, and vice versa. It has been proposed that the vulnerability of environmental and genetic factors have to be present for the traumatic experience to trigger the onset of OCD [32,53,63].Frías-Ibáñez et al. (2013) discussed a clinical case of a 19-year old woman who developed a comorbidity between disorders: OCD, PTSD, Dissociative unspecified, and obsessive-compulsive personality traits; after suffering sexual abuse at 13 years of age. Symptoms reported include a compulsion to wash hands (1-3 hours a day) from physical contact with men, and musical obsessions over a 12-year period [58]. Zabalza-Estévez (2014) included a clinical study of a 72-year old woman who suddenly began to evoke songs that she had heard in her childhood, and sporadically, the sound of ambulances during the attack of the Nazi troops in Leningrad [1]. Both clinical cases can be explained from the theory of Rachman (1997) and Sasson et al. (2004), which relate the exposure of traumatic events to obsessive thoughts that are generalized to other life experiences [64,65].
3.4.3. OCD: Musical Obsessions and Hallucinations in Schizophrenia
- Clinical studies have revealed higher comorbidity rates for OCD in the schizophrenia population than was previously acknowledged [66]. Clinically significant prevalence rates of obsessive-compulsive symptoms have been reported in the schizophrenia population (10% to 52%), while OCD in the schizophrenia population is (7.8% to 26%) [66]. Still, some of these comorbid OCD cases were due to prescribed drugs for treatment management. Studies with community samples showed a vulnerability of 50% to 60% of a subject diagnosed with OCD to suffer from schizophrenia. A relationship between these two disorders has been found that ranges from 12%-15% of patients with primary diagnosis of the psychotic spectrum. This represents a prevalence six times higher than the general population [58].
3.4.4. OCD: Musical Obsessions and Hallucinations in Mood Disorders
- Research shows a close clinical relationship between depressive pathology and OCD. Many OCD subjects become depressed and comorbidity has relevant clinical implications [49]. Epidemiological studies of OCD patients revealed lifetime depression rates of 73.4% and 81.2% in early and late onset cases [49,67]. 30% of OCD patients meet criteria for a depressive disorder [52]. It is relevant to address the complexity of distinguishing primary obsessions from secondary ones in the context of a depressive disorder. In a percentage not greater than 10% of patients, obsessive and depressive symptoms occur concurrently [52]. Fontela-Vivanco et al. (2012) and Welner et al. (1976) concluded that a diagnosis of primary OCD with secondary depressive symptoms is characterized by an early onset, longer duration, and fewer remissions. In contrast, a primary affective diagnosis has a more episodic course and the person has a long history of affective disorders [52,68]. Consistent with previous research, the results of a study of patients 18 to 45 years old, with OCD comorbidity and bipolarity showed a prevalence of 21% in bipolar disorder and 12% in unipolar disorder [52]. The clinical case of a 48-year old woman diagnosed with Bipolar II Disorder and obsessive personality traits reported that she listened to songs almost all day before starting the hypomania phase, as a warning of relapse [8].
3.4.5. OCD: Musical Obsessions and Hallucinations in Personality Disorders
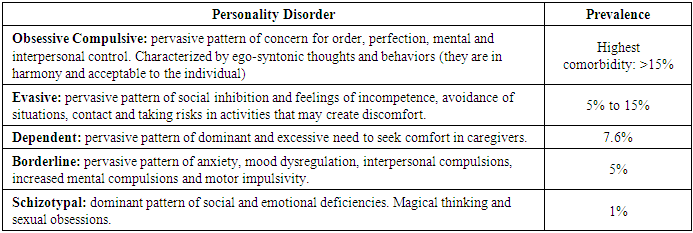
- OCD comorbidity and personality disorders are common. It represents a higher risk (52%) for OCD patients of developing at least one comorbid personality disorder [69]. The prevalence of comorbidity of personality disorders and OCD [69,70,71,72,73,74,75,76] is exhibited in Table 1.
|
3.5. Neurobiology of Musical Obsessions and Hallucinations
- There is little knowledge related to the pathophysiology of musical obsessions, and few studies have addressed the pattern of neurobiological activation [2,5]. In the research by Zungu-Dirwayi et al. (1999), a single photon emission tomography (SPECT) was performed in two patients with musical obsessions. A reduction in cerebral blood flow was found in temporal and frontal lobes [6]. In other neurobiological conditions the present musical hallucinations, such as musicogenic epilepsy and musical illusion, brain dysfunction was associated with the temporal lobe [2,77,78]. Contributions in neuroimaging studies suggest neuroanatomical correlations of some mental disorders, such as depression, schizophrenia, obsessive-compulsive, and others mentioned previously [79,80].
3.5.1. Neurobiology of OCD
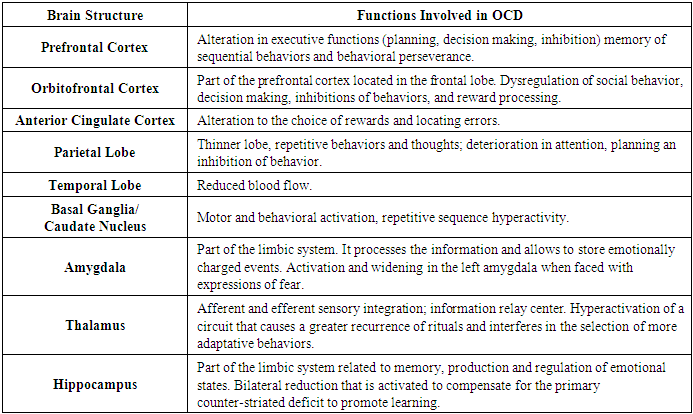
- Research indicates that OCD is a neurobiological disorder that results from the combination of biological, genetic, cognitive, and environmental factors [39]. There are several theories that relate some brain areas with the production of obsessive-compulsive symptoms. However, discrepancies continue in the conceptualization of a standardized neurobiological model [41,79]. Several authors have proposed the neurobiological model of OCD that links the cortico-striatal-thalamic circuit, considered critical for the phenomenology of the disorder and the processing of information. These explain that within the circuit there is an imbalance between direct (excitation) and indirect (inhibition) pathways. Brain blockage in the caudate nucleus and hyperactivation between the thalamus and the orbitofrontal cortex results in a compromise of the four lobes. Overstimulation could cause a disinhibition of the behavioral impulse that contributes to intrusive thoughts and repetitive behaviors [55,79,81,82]. According to other researchers, functional neuroimaging studies of OCD patients show changes in the anterior cingular cortex and reduction in cortical thickness in various areas, which may contribute to disinhibition and damage to the basal ganglia [11,42,81,83,84]. Among other contributors, the influence of neurochemical theory on the functional role of neurotransmitters such as serotonin, dopamine, GABA and glutamate, has been found when compared to healthy subjects [2,5,12,39,7985,86]. The neurobiology of TOC and its functions are described in Table 2 [81.86.87,88,89].
|
3.5.2. Neurobiology of PTSD
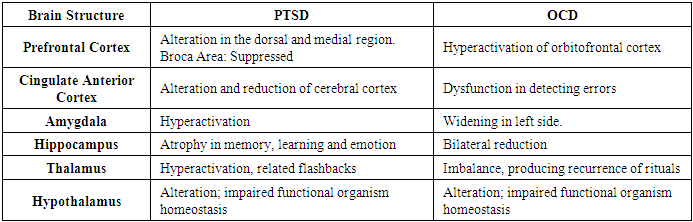
- PTSD presents individual variations that depend on genetic, neurobiological, neuropsychological factors, environmental influences and epigenetic changes [92,93]. In recent decades, it has been found that its underlying neurobiological processes are not fully understood [93,94,95]. It is evident that in PTSD, the hypothalamic-pituitary-adrenal axis (HPA) is altered [80,93,96]. At the neurocognitive level, patients show alteration of executive functions (attention, planning, working memory, among others) and the dysregulation of mood. The neurobiology of PTSD and OCD is exhibited in Table 3 [96,97,98.99].
|
3.5.3. Neurobiology of Schizophrenia
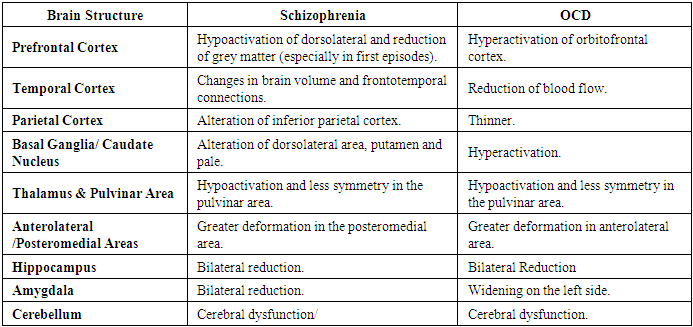
- Patients with OCD and schizophrenia, called schizo-obsessive, show a pattern of neurobiological dysfunction, which explains the simultaneous expression of symptoms [66]. There are enough studies done on the neuropsychological basis of OCD and schizophrenia. Consistency in the contribution of serotonin and dopamine as the main neurotransmitters has been demonstrated in both disorders [66]. These pathologies present anomalies in the fronto-striatal circuits and structures, demonstrating a marked convergence and alterations in the thalamus [58,100]. However, other authors explain that cortical dysfunction in schizophrenia is more complicated and can be described as a loss of synchrony between brain functions [100,101]. The neurobiological comparison of schizophrenia and OCD is established in Table 4 [89,100,102,103,104,105,106,107,108,109,110].
|
3.5.4. Neurobiology of Depression and Personality Disorders
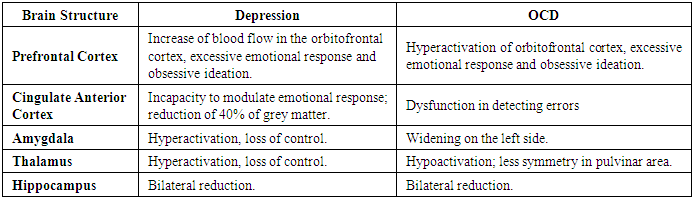
- Neuroimaging studies have found altered brain structures, both in depression and personality disorders, specifically schizotypal. In relation to schizotypal disorder, alterations in the orbitofrontal and dorsolateral cortex have been evidenced [69]. In OCD there are also changes in the orbitofrontal cortex, which explains the lack of control and mood dysregulation in both disorders. The neurobiology of depression and OCD is described in Table 5 [69,80,111].
|
3.5.5. Neurobiology of Other Disorders
- The hyperactivity of the peripheral auditory system associated with Meniere’s disease (rhythmic tinnitus) suggests that musical obsessions have an otological basis [5,6]. Musical hallucinations caused by neurological disorders have been associated with lesions in the brainstem or in one of the two hemispheres, generally the right [31,77]. Furthermore, in patients with strokes and degenerative diseases such as dementia, it rarely occurs, and consists of a musical desire [26,112,113]. However, Stewart et al. (2006) presented a case of a patient with Alzheimer’s disease who reported musical hallucinations, observing an increase in perfusion of the temporal lobe and cingulate gyrus [34]. Musical hallucinations are rarely associated with epileptic foci and problematic substance use [31]. Nevertheless, other studies have associated musical hallucinations with acute use of action drugs in the central nervous system [34].In a study of 666 patients with temporal lobe epilepsy, 16% had auditory hallucinations, and seizures were associated with activation of the right superior temporal gyrus [26,114]. It has been suggested that in some cases an electroencephalogram may be useful in differentiating a musical obsession or hallucination from a complex partial seizure. Furthermore, the use of imaging can rule out whether musical obsessions or hallucinations are due to brain injury (tumor), especially in patients who have a sudden onset [5].
3.6. Psychological Treatments and Pharmacotherapy
- Research has established cognitive behavioral therapy (CBT) as the first line of evidence-based psychotherapeutic approach. It is considered to be the most effective for the treatment of musical obsessions, which are mostly manifested in patients diagnosed with OCD and other related disorders. Through the therapeutic approach, patients are exposed to the stimulus that caused anxiety, learn to inhibit the response of compulsive behavior, and acquire more adaptative behaviors and coping strategies. It has been established that combining this intervention methodology with psychoeducation, gradual exposure, stress inoculation, along with couple and family therapy, has proven highly effective in the remission of symptoms. For example, women during pregnancy or postpartum, and in comorbid conditions such as those previously discussed throughout the article. Additionally, it has been shown that the inclusion of motivational therapy in these practices can be effective in increasing the patient’s commitment to the therapeutic process [1,2,5,11,39,43,47,58,81,103]. Pharmacotherapy integrated with psychological interventions show a reduction of symptoms between 40% and 60%. The treatment may take a period of four to 10 weeks to reflect a significant change in symptoms. The literature mentioned the effectiveness of deep brain stimulation for patients who present a severe resistance to treatment. Nevertheless, it has only been studied in a small number of patients, since it is considered to be a last resort treatment due to limited approval by the FDA [2,8,11,39].
4. Conclusions
- Music has an important role in people’s lives. It usually evokes emotions and memories related to an individual’s history. However, music becomes a problem when it takes on a life of its own. It can become an intrusive and invasive entity, which becomes an obsession or musical hallucination, with repercussions on the quality of life of those who experience it. Musical obsessions and hallucinations are symptoms that generally go unnoticed, both by the health professionals and the patients. On many occasions, the comorbidity of symptoms between disorders and the patient’s failure to recognize and report symptoms contribute to a counterproductive approach to diagnosis, management, and treatment. In order to establish a differential diagnosis, it is necessary to understand individual and gender differences, and to know the underlying neurobiology of mental, neurological and organic disorders.
5. Clinical Recommendations
- According to the findings in the literature review, some clinical recommendations are made. A good collaborative alliance must be established between the patient and health professionals. In this way, a safe environment and therapeutic motivational commitment are promoted. This allows exploring individual differences in the patient’s life history, and not omitting relevant information that could impact establishing an accurate diagnosis. Taking into account the relevance in the collection of developmental history data, helps to identify factors of organic and psychological vulnerability in the patient. The high comorbidity between diagnoses highlights the imperative need to establish a primary differential diagnosis. The similarity of the symptoms could overlap the causal origin of the disorder, resulting in detrimental consequences that significantly impact the patient’s well-being. It has been evidenced that psychoeducation is an important tool that provides patients and their support networks with information on the chronic nature of their diagnosis and contributes to the reduction of symptoms. Furthermore, it allows them to explore alternatives that are attuned to their reality, and know what to expect in response to treatment and its effectiveness in promoting a better quality of life. Finally, health professionals could consider and include the role of music in their patient’s lives as part of data collection in future interventions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML