-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Psychology and Behavioral Sciences
p-ISSN: 2163-1948 e-ISSN: 2163-1956
2014; 4(6): 208-214
doi:10.5923/j.ijpbs.20140406.04
Age-Related Deficits on the Trail-Making Test Part A: Separating Specific Deficits of Visual Search from Generalized Sensorimotor Slowing
Christine Kirchner, Ina Völker, Otmar L. Bock
Institute of Physiology and Anatomy, German Sport University, Cologne, Germany
Correspondence to: Christine Kirchner, Institute of Physiology and Anatomy, German Sport University, Cologne, Germany.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Trail-Making Test part A (TMT-A) is sensitive to the speed of perceptual processing, the speed of motor programming and the proficiency of visual search. Here we explore why, in previous work, performance on TMT-A was found to decay in old age. We (1) distangle the contribution of visual search from that of basic perceptuo-motor processing by implementing a control version of TMT in which visual search is minimized, (2) investigate the role of task experience by embedding TMT in a context that models daily workplace activities of some but not other participants and (3) explore the age deficits on TMT-A at the level of eye movements. Ten young adults (YA), ten older adults without task experience (OA), as well as ten older adults with task experience (OE) participated in a barcode scanning task in which numbered “parcels” had to be scanned in ascending order. The “parcels” were placed in a regular, easily predicable order for TMT-C, but in a mixed order for an unspeeded and a speeded version of TMT-A. We found no group differences for completion time and gaze pattern in TMT-C. Completion time increased and the gaze pattern changed accordingly in TMT-A, and changes were more pronounced in OA and OE compared to YA. Specifically, older persons had a longer completion time, their gaze took longer to move from one scanned parcel to the next, but their gaze didn’t rest longer on a scanned parcel. The differential effects of old age on TMT-A versus TMT-C, and the effects of age on the gaze pattern in TMT-A, provide converging evidence that age-related slowing on TMT-A was related to specific deficits of visual search, not to generalized deficits of sensorimotor processing. Slowing on TMT-A was not modified by instructions (speeded versus unspeeded), nor by decades of work-related experience on a similar task.
Keywords: Eye movements, Gaze path, Trail-Making Test, Visual search, Aging
Cite this paper: Christine Kirchner, Ina Völker, Otmar L. Bock, Age-Related Deficits on the Trail-Making Test Part A: Separating Specific Deficits of Visual Search from Generalized Sensorimotor Slowing, International Journal of Psychology and Behavioral Sciences, Vol. 4 No. 6, 2014, pp. 208-214. doi: 10.5923/j.ijpbs.20140406.04.
1. Introduction
- The Trail-Making Test is a well-established, expedient and widely used cognitive assessment tool. In TMT part A, subjects are asked to connect randomly distributed numbers in an ascending order; in TMT part B, they are required to connect randomly distributed letters and numbers in an alternating, ascending order (1, A, 2, B, 3, …). In either case, the outcome measure is completion time. Converging evidence from many studies suggests that individual differences on TMT-A are mainly attributable to the speed of perceptual processing, the speed of motor programming and the proficiency of visual search; differences on TMT-B are additionally related to working memory and task switching abilities [e.g.; 1]. A number of studies reported that performance on the Trail-Making Test decays in old age. Deficits are more pronounced on TMT-B, but even on TMT-A, 40-59 year olds are about 17% to 25,5% slower than healthy 20-29 years olds [2-5]. The present study evaluates possible reasons for the age-related slowing on TMT-A. In fact, all three determinants described by Sanchez-Cubillo et al. [1] are degraded in old age and thus may contribute to age-related slowing on TMT-A: Compared to young adults, older persons exhibit a lower processing speed [6, 7], both at the perceptual [8-10] and at the motor level [11-13] and a poorer visual-search ability, particularly when complex visual scenes are presented [14-16]. The latter deficit was attributed to difficulties in the spatial localization of task-relevant information [17], an increased sensitivity to distracting stimuli [16, 18, 19; see however 20] and problems of attentional control [21, 22; see however 15]. It has also been shown that in visual search, older individuals rely more heavily than young ones on contextual information [23, 24]. It therefore is conceivable that age-related deficits are ameliorated when visual search is embedded in a familiar context. To distangle the contribution of visual search from that of basic perceptuo-motor processing in TMT-A, we implemented a version of the Trail-Making Test in which visual search is minimized. In this TMT-C, numbers are laid out in an easily predictable sequence, from left to right line by line, as in reading. Impaired perceptuo-motor processing should manifest both on TMT-C and on TMT-A, while impaired visual search should manifest on TMT-A alone.A second purpose of our study was to analyse older person’s deficits on TMT-A at the level of eye movements. We are not aware of earlier work on age-related changes of the gaze pattern during the Trail-Making Test, but changes have been observed with other paradigms. Compared to young adults, older participants execute more fixations [25, 26] with longer fixation durations [27, 28], look at critical upcoming locations earlier and longer [29-32], have problems to suppress undesired saccades [33-35] and – although they have no difficulties to disengeage their gaze from inspected locations [36, 37] – they return their gaze to such locations more often [26, 38]. We therefore expected that during TMT-A as well, older participants will have no problems to detach their gaze from the last-inspected target but will refixate that target more often, will execute more fixations with longer durations before attaching their gaze to the next target, and look at that target for a longer time.Trail-Making Tests have been administered in a number of variants besides the standard written version, e.g., in an oral [2], an eyes-only [39], a keyboard [40] and a walking version [41]. Here we introduce a new version in which trail-making is embedded in a barcode scanning task, a typical activity for workers in a goods receiving department. This allowed us to compare older persons with daily experience on this task to older persons without such experience. We hypothesized that by embedding the test in a familiar context, we will facilitate performance of the experienced relative to the unexperienced older group.
2. Methods
- Thirty participants (19 women, 11 men) were recruited for the study. Ten were young adults with no particular experience in barcode scanning (YA, 22,5 ± 2,42 years of age), ten were older adults with no particular experience in barcode scanning (OA, 52,5 ± 6,29 years of age) and the remaining ten were older employees from the goods receiving department of a wholesale company who had 24,55 ± 7,93 years of professional experience that included barcode scanning for about 3 hours per day (OE, 50,2 ± 7,77 years of age). The daily activities of all three groups were composed of a combination of physical and cognitive tasks. At the time of testing, no subject reported any orthopedic or mental disease, and had normal vision. All participants gave their written informed consent. The study was approved by the local Ethics Committee of the German Sport University.Participants stood in front of a poster (31,1 inch horizontal x 46,8 inch vertical) on which 20 rectangles of four different sizes were drawn (9,25 x 5,12 inch, 11,61 x 6,69 inch, 4,33 x 5,12 inch, 5,12 x 9,25 inch). Each rectangle contained a barcode (3,94 x 1,57 inch) and a number (1 to 20) to the left of it. This poster layout is illustrated in Fig. 1. Participants were told that the rectangles represent “parcels” whose barcodes they have to scan with a handheld scanner (Albasca MK-1000ZB, as used at work by OE). They held the scanner in their dominant right hand, scanned first the parcel numbered “1”, and proceed in ascending order until they scanned parcel number “20”. The use of the scanner was explained to unexperienced subjects beforehand. All participants were able to reach all barcodes solely through arm and trunk movements.
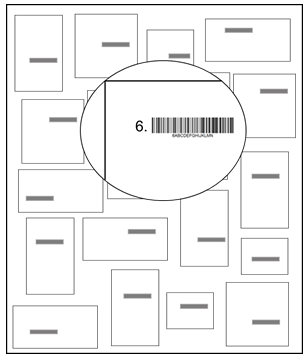 | Figure 1. Illustration of a poster with the barcodes |
3. Results
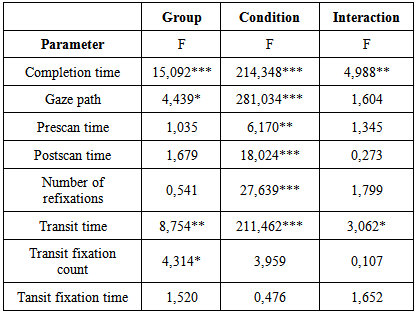
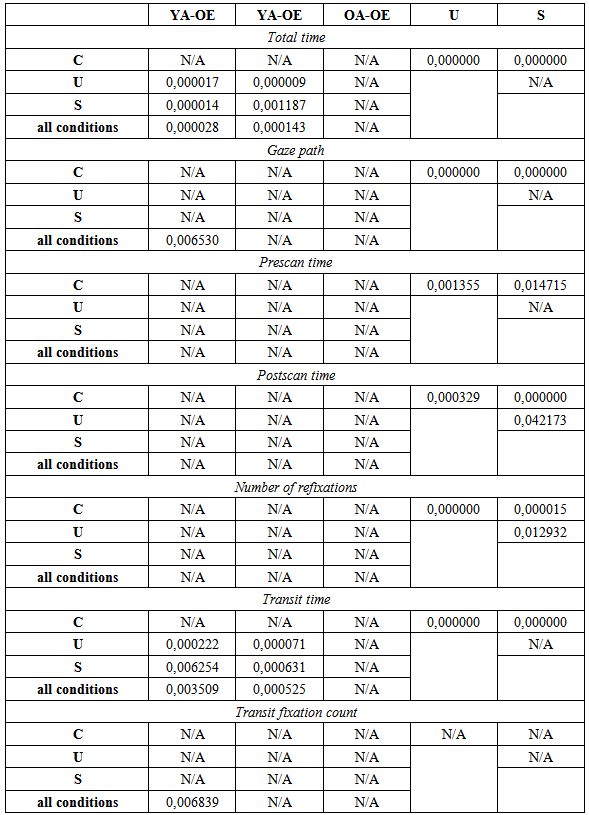
- Table 1 summarizes the ANOVA outcome for all dependent variables, and Table 2 the post-hoc decompositions of the pertinent significant effects of Condition (last two columns), Group (last row for each variable) and Condition*Group (remaining cells). One variable, transit fixation time, yielded no significance at all. Three variables yielded significance for Condition only; they are illustrated in Fig. 2. The remaining four variables yielded significance for Group, and two of them also for Condition*Group; they are depicted in Fig. 3.
|
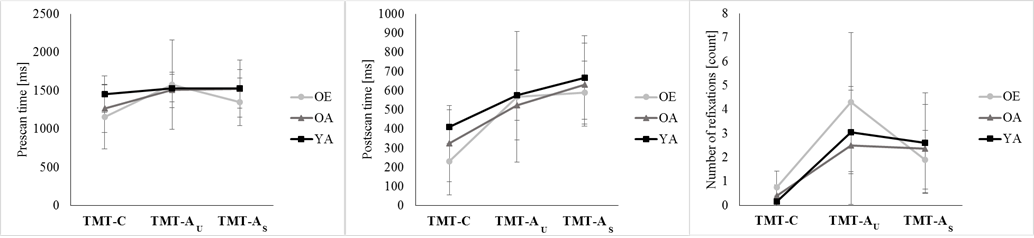 | Figure 2. Means of three dependent variables for the three TMT Conditions and the three Groups (OE, OA YA). Error bars are standard deviations |
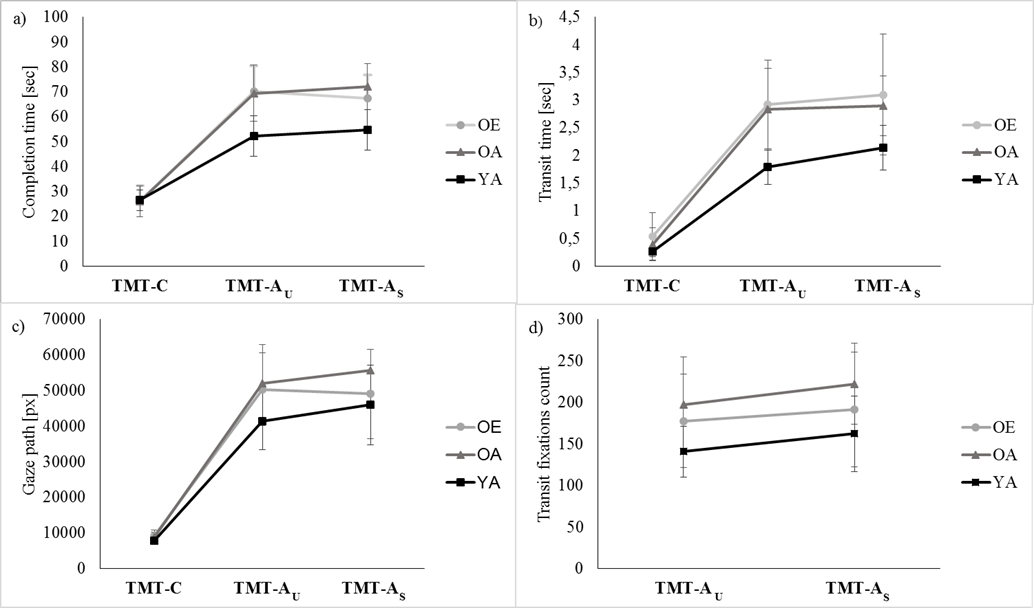 | Figure 3. Means scores of four dependent variables, separately for each group and condition. Error bars are standard deviations |
4. Discussion
- Our study evaluates whether age-related decrements of TMT-A are modulated by task experience, and to what extent they reflect specific deficits of visual search rather than generalized sensorimotor slowing. To this end, we analyze not only the standard outcome measure, total completion time, but also include measures of the subjects’ gaze pattern.We implemented a control condition which minimized visual search, TMT-C, and found no evidence for an effect of age or experience on completion time or gaze. This might seem surprising, given that reports on age-related sensorimotor slowing [11, 6, 7] and changes of the gaze pattern (e.g., 26, 27, 30-33] abound in literature. One possible explanation is that the mean age of our older group was only about 50, compared to 60 – 70 in most earlier studies. When data from other studies (e.g., 42-44] are interpolated to predict sensorimotor speed at the age of 50 – capitalizing on the fact that speed declines in a close-to-linear fashion from the age of 20 on [45, 46] – the expected decrement is about 15%. In contrast, we observed no decrement at all for completion time in TMT-C. It therefore is unlikely that our older subjects were too young to exhibit noticeable sensorimotor slowing, and we rather propose an alternative interpretation for our findings: TMT-C might be resistant to sensorimotor slowing since it is a more natural task than the abstract laboratory paradigms in the above studies. We submit that all persons, even if they don’t routinely scan barcodes at work, regularly observe this activity e.g. in supermarkets and department stores. Indeed, there is experimental evidence that age-related slowing is absent in familiar, ecologically valid scenarios: no age deficits were observed when a reaction-time task was embedded in a realistic car-driving scenario, where subjects had to brake in response to unexpected traffic events [47-49]. Summing up, TMT-C might be resistant to sensorimotor slowing since it is more natural than the abstract laboratory paradigms typically used in literature.TMT-C served as a control for the unspeeded and speeded variants of TMT-A, which additionally required visual search. Not surprisingly, this additional requirement led to a poorer performance on all registered variables: the gaze travelled along a longer path, rested longer on each scanned parcel and returned to it more often, spent more time between scanned parcels and as a consequence, completion time increased. Interestingly, these changes were comparable in TMT-AU and TMT-AS, except for two minor differences: in the latter condition, subjects’ gaze remained longer in the scanned parcel and returned to it less often. These differences, however, had no noticeable effect on completion time. In other words, we were unable to speed up visual search by means of verbal instructions.The increase of completion time from TMT-C to TMT-A was much more pronounced in our older than in our young adults, which confirms earlier accounts about age-related slowing on TMT-A [2-5]. More importantly, the emergence of slowing on TMT-A but not on TMT-C suggests that the underlying deficit is related specifically to visual search rather than to sensorimotor processing in general. This conclusion is supported by our gaze data: compared to young adults, older persons didn’t increase their pre-and postscan time but their transit time was 0,899 s longer, which adds up to 17,081 s for 19 transits between 20 parcels and thus fully accounts for the 17,371 s increase of completion time. Older participants therefore spent more time looking for the next to-be-scanned parcel, but not fixating the presently scanned parcel. This prolonged search for the next parcel is paralleled by an increased length of the gaze path and an increased number – but not duration – of fixations.The observed changes of the gaze pattern in old age fit well with earlier work which reported that during visual search, older participants execute more fixations [25, 26] with no increase of fixation duration [26] unless complex visual processing is required [27, 28]. As in our study, the number of refixations was unchanged in old age [18], or it was found to increase [38, 26]. Studies in which subjects had to negotiate obstacles while walking found that elderly persons’s gaze is directed at critical locations earlier and longer [29-32]; this increase was not reflected by a longer pre- or postscan time in our study, possibly because scanning was not relevant for posture and therefore didn’t call for in-depth processing. Summing up, our findings on age-related changes of the gaze pattern during visual search are consistent with literature.Our study implemented a TMT variant which models the working-life experience of employees in a goods receiving department: the search for numbers was embedded in a barcode scanning task, and rather than drawing lines, subjects operated a scanner type which group OE actually used in their daily work. We expected that subjects from group OE would benefit from their working-life experience and perform better than those from group OA; however, such benefits didn’t emerge for completion time or any gaze parameter. It therefore appears that decades of experience with parcel scanning didn’t improve sensorimotor processing (TMT-C) or visual search (TMT-A minus TMT-C) in our experiment. One possible explanation is that OE felt uncomfortable to call up performance similar to their daily work, such that the benefits of experience were cancelled by the costs of distress. As an alternative explanation, our variant of TMT was not sufficiently similar to the daily work of OE, which demands not only scanning, but also simultaneous activities such as sorting, reading or checking the content of a parcel against an invoice. In any case, our findings provide no evidence that age-related deficits of visual search can be ameliorated by extensive training on similar tasks.Taken together, our study confirmed that completion time on TMT-A increases in old age, attributed this increase to specific deficits of visual search rather than to generalized sensorimotor slowing, and documented the correlates of those deficits at the level of eye movements. We found no evidence that age-related deficits on TMT-A are reducible by practice.
ACKNOWLEDGEMENTS
- Thanks are due to all participants and student assistants Kristin Henkelmann and Denise Schulze.
References
| [1] | Sanchez-Cubillo, I.; Periánez, J.A., Adrober-Roig, D., Rodriguez-Sánchez, J.M., Rios-Lago, M., Tirapu, J. and Barcelo, F. (2009). Construct validity of the Trail Making Test: Role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. Journal of the International Neuropsychological Society, 15: 438 – 450. |
| [2] | Ricker, J.H. & Axelrod, B.N. (1994). Analysis of an oral paradigm for the Trail Making Test. Psychological Assessment Resources, 1 (1): 47-51. |
| [3] | Tombaugh, T.N. (2004). Trail Making Test A and B: Normative data stratified by age and education. Archives of Clinical Neuropsychology, 19: 203-214. |
| [4] | Kennedy, K.J. (1981). Age effects on Trail Making Test performance. Perceptual and Major Skills, 52: 671-675. |
| [5] | Goul, W.R. & Brown, M. (1970). Effects of age and intelligence on trail making test performance and validity. Perceptual and Motor Skills, 30: 319-326. |
| [6] | Cerella, J. (1985). Information processing rates in the elderly. Pschol Bull. 98(1): 67-83. |
| [7] | Robitaille, A., Muniz, G., Piccinin; A.M., Johansson, B. & Hofer, S.M. (2012). Multivariate Longitudinal Modeling of Cognitive Aging: Associations Among Change and Variation in Processing Speed and Visuospatial Ability. GeroPsych. 25(1): 15-24. |
| [8] | Walsh, D.A. (1976). Age differences in central perceptual processing: a dichoptic backward masking investigation. J Gerontol. 31(2): 178-85. |
| [9] | Salthouse T.A. (2000). Aging and measures of processing speed. Biol Psychol. 54(1-3):35-54. |
| [10] | Ritchie, S. J., Tucker-Drob, E.M. & Deary, I.J. (2014). A strong link between speed of visual discrimination and cognitive ageing. Curr Biol. 24(15): 681-683. doi: 10.1016/j.cub.2014.06.012. |
| [11] | Salthouse, T.A. (1996). The processing-speed theory of adult age differences in cognition. Psychol Rev., 103(3): 403-28. |
| [12] | Stelmach, G.E., Amrhein, P.C. & Goggin, N.L. (1988). Age differences in bimanual coordination. J Gerontol. 43(1): 18-23. |
| [13] | Sleimen-Malkoun, R., Temprado, J.J. & Berton, E. (2013). Age-related changes of movement patterns in discrete Fitts' task. BMC Neurosci. 14:145. doi:10.1186/1471-2202-14-145. |
| [14] | Scialfa, C.T., Esau, S.P. & Joffe, K.M. (1998). Age, target-distractor similarity, and visual search. Exp Aging Res. 24(4): 337-58. |
| [15] | Madden, D.J., Gottlob, L.R. & Allen, P.A. (1999). Adult age differences in visual search accuracy: attentional guidance and target detectability. Psychol Aging. 14(4): 683-94. |
| [16] | Hommel, B., Li, K.Z. & Li, S.C. (2004). Visual search across the life span. Dev Psychol. 40(4): 545-58. |
| [17] | Owsley, C., Burton-Danner, K. & Jackson, G.R. (2000). Aging and spatial localization during feature search. Gerontology. 46(6): 300-305. |
| [18] | Kramer, A.F., Hahn, S., Irwin DE, Theeuwes J. (2000). Age differences in the control of looking behavior: do you know where your eyes have been? Psychol Sci. 11(3): 210-207. |
| [19] | Hasher, L., Stoltzfus, E.R., Zacks, R.T. & Rypma, B. (1991). Age and inhibition. J Exp Psychol Learn Mem Cogn. 17(1): 163-169. |
| [20] | Ho, G., Scialfa, C.T., Caird, J.K. & Graw, T. (2001).Visual search for traffic signs: the effects of clutter, luminance, and aging. Hum Factors. 43(2): 194-207. |
| [21] | Rabbitt, P. (1965). An Age-decrement in the ability to ignore irrelevant information. J Gerontol. 20: 233-238. |
| [22] | Sekuler, R. & Ball, K. (1986). Visual localization: age and practice. J Opt Soc Am A. 3(6): 864-867. |
| [23] | Becic, E., Kramer, A.F. & Boot, W.R. (2007). Age-related differences in visual search in dynamic displays. Psychol Aging. 22(1): 67-74. |
| [24] | Neider, M.B. & Kramer, A.F. (2011). Older adults capitalize on contextual information to guide search. Exp Aging Res., 37(5): 539-571. doi: 10.1080/0361073X.2011.619864. |
| [25] | Grahame, M., Laberge, J. & Scialfa, C.T. (2004). Age differences in search of web pages: the effects of link size, link number, and clutter. Hum Factors. 46(3): 385-98. |
| [26] | Maltz, M. & Shinar, D. (1999). Eye movements of younger and older drivers. Hum Factors. 41(1): 15-25. |
| [27] | Porter, G., Tales, A., Troscianko, T., Wilcock, G., Haworth, J. & Leonards, U. (2009). New insights into feature and conjunction search: I. Evidence from pupil size, eye movements and ageing. Cortex. 46(5): 621-36. doi: 10.1016/j.cortex.2009.04.013. |
| [28] | Buscher, G., Dumais, S., & Cutrell, E. (2010). The good, the bad, and the random: An eye-tracking study of ad quality in web search. In SIGIR 2010 (pp. 42-49). Geneva, Switzerland: ACM Press. |
| [29] | Di Fabio, R.P., Zampieri, C. & Greany, J.F. (2003). Aging and saccade-stepping interactions in humans. Neurosci Lett. 339(3): 179-182. |
| [30] | Chapman, G.J. & Hollands, M.A (2005). Evidence for a link between changes to gaze behaviour and risk of falling in older adults during adaptive locomotion. Gait Posture. 24(3): 288-294. Epub 2005 Nov 9. |
| [31] | Keller Chandra, S., Bockisch, C.J., Dietz, V., Hegemann, S.C., Straumann, D. & Van Hedel, H.J. (2011). Gaze strategies for avoiding obstacles: Differences between young and elderly subjects. Gait Posture. 34(3):340-346. doi: 10.1016/j.gaitpost.2011.05.022. Epub 2011 Jun 22. |
| [32] | Zietz, D. & Hollands, M. (2009). Gaze Behavior of Young and Older Adults During Stair Walking. Journal of Motor Behavior. 41(4): 357–365. |
| [33] | Butler, K.M., Zacks, R.T. & Henderson, J.M. (1999). Suppression of reflexive saccades in younger and older adults: Age comparisons on an antisaccade task. Memory & Cognition. 27: 584–589. |
| [34] | Harsay, H.A., Buitenweg, J.I.V, Wijnen, J.G., Guerreiro, M.J.S. & Ridderinkhof, K.R. (2010). Remedial effects of motivational incentive on declining cognitive control in healthy aging and Parkinson’s disease. Front Aging Neurosci. 2: 144.doi: 10.3389/fnagi.2010.00144. |
| [35] | Beurskens, R. & Bock, O. (2012). Age-related decline of peripheral visual processing: the role of eye movements. Exp Brain Res. 217(1): 117–124. doi:10.1007/s00221-011-2978-3. |
| [36] | Pratt, J., Abrams, R.A. & Chasteen, A.L. (1997). Initiation and inhibition of saccadic eye movements in younger and older adults: an analysis of the gap effect. J Gerontol B Psychol Sci Soc Sci. 52: 103–107. |
| [37] | Munoz, D.P., Broughton, J.R., Goldring, J.E. & Armstrong, I.T. (1998). Age-related performance of human subjects on saccadic eye movement tasks. Exp Brain Res. 121: 391–400. |
| [38] | Veiel, L.L., Storandt, M. & Abrams, R.A. (2006). Visual Search for Change in Older Adults. Psychology and Aging. 21(4): 754–762. doi: 10.1037/0882-7974.21.4.754. |
| [39] | Hicks, S.L., Sharma, R., Khan, A.N., Berna, C.M., Waldecker, A., Talbot, K., Kennard, C., Turner, M.R. (2013). An eye-tracking version of the trail-making test. PLoS One. 8(12). doi: 10.1371/journal.pone.0084061. |
| [40] | Salthouse, T. A., Fristoe, N. M. (1995). Process analysis of adult age effects on a computer-administered Trail Making Test. Neuropsychology, 9(4): 518-528. doi: 10.1037/0894-4105.9.4.518. |
| [41] | Alexander, N.B., Ashton-Miller, J.A., Giordani, B., Guire, K. & Schultz, A.B. (2005). Age differences in timed accurate stepping with increasing cognitive and visual demand: a walking trail making test. J Gerontol A Biol Sci Med Sci. 60 (12): 1558–1562. |
| [42] | Langan, J., Peltier, S.J., Bo, J., Fling, B.W., Welsh, R.C., Seidler, R.D. (2010). Functional implications of age differences in motor system connectivity. Front Syst Neurosci; 4:17. doi: 10.3389/fnsys.2010.00017. eCollection 2010. |
| [43] | Yordanova, J., Falkenstein, M., Hohnsbein, J., Kolev, V. (2004). Parallel systems of error processing in the brain. Neuroimage, 22: 590–602. |
| [44] | Van der Lubbe, R.H.J., Verleger, R. (2002). Aging and the Simon task. Psychophysiology, 39, pp 100-110. DOI: 10.1017.S0048577201020042. |
| [45] | Schaie, K.W. (1989). Perceptual speed in adulthood: Cross-sectional and longitudinal studies. Psychology and Aging, 4(4): 443-453. |
| [46] | Spirduso, W.W. (1995). Physical Dimension of Aging. Champaign: Human Kinetics. |
| [47] | Lerner, N.D. (1993). Brake perception-reaction times of older and younger drivers. Proceedings of the Human Factors and Ergonomics Society 37th Annual Meeting, 206-210. DOI: 10.1177/154193129303700211. |
| [48] | Warshawsky-Livne, L., Shinar, D. (2002). Effects of uncertainty, transmission type, driver age and gender on brake reaction and movement time. J Safety Res., 33(1): 117-28. |
| [49] | Olson, P.L., Siwak, M. (1986). Perception-response time to unexpected roadway hazards. Human Factors, 28: 91- 96. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML