-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Psychology and Behavioral Sciences
p-ISSN: 2163-1948 e-ISSN: 2163-1956
2013; 3(5): 123-130
doi:10.5923/j.ijpbs.20130305.02
Spatiotemporal Cortical Activation Underlying Reward Processing under “Worse off than Some, Better off than Many” Comparison: An ERP Study
Kangcheng Wang1, 2, Junyi Yang1, 2, Jiang Qiu1, 2
1Key Laboratory of Cognition and Personality (SWU), Ministry of Education, Chongqing, China
2Faculty of Psychology, Southwest University, Chongqing , 400715, China
Correspondence to: Jiang Qiu, Key Laboratory of Cognition and Personality (SWU), Ministry of Education, Chongqing, China.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Event-related potentials (ERPs) were recorded to explore the electrophysiological correlates of reward processing in the complex social comparison context. Four social comparison stimulus categories (four relative reward levels) were mainly prepared: Self:He:She = 1:2:4 (Disadvantageous Inequity); Self:He:She = 1:1:1 (Equity); Self:He:She = 4:2:1 (Advantageous Inequity); Self:He:She = 2:1:4 (Ambiguous Comparison: worse off than some, better off than many). Results showed that: Disadvantageous, Advantageous Inequity and Ambiguous Comparison conditions all elicited a more negative ERP deflection (N350-550) than did Equity between 350-550 ms, which might reflect reward prediction error. Then, Ambiguous Comparison, Disadvantageous and Advantageous Inequity all elicited a more ERP deflection than did Equity between 550-750 ms, which might be involved in evaluating the rewards after detection of reward prediction error. Furthermore, Ambiguous Comparison elicited a greater negativity (N550-750) than did Disadvantageous and Advantageous Inequity, and the generator of N550-750 was localized in the caudate nucleus and the anterior cingulate cortex (ACC), which might be related to reward processing and cognitive conflict controlling. Last, Disadvantageous Inequity elicited a late more positive ERP component (LPC) than did Advantageous Inequity, Equity and Ambiguous Comparison conditions between 1200-1400 ms. The LPC might reflect strong unpleasant emotional experience (social comparison affects subjective well-being).
Keywords: Social Comparison, Reward Processing, Caudate Nucleus, Subjective Well-being, Event-related Potentials (ERPs)
Cite this paper: Kangcheng Wang, Junyi Yang, Jiang Qiu, Spatiotemporal Cortical Activation Underlying Reward Processing under “Worse off than Some, Better off than Many” Comparison: An ERP Study, International Journal of Psychology and Behavioral Sciences, Vol. 3 No. 5, 2013, pp. 123-130. doi: 10.5923/j.ijpbs.20130305.02.
Article Outline
1. Introduction
- Social comparison profoundly affects reward processing (Reward processing posits a very specific computational challenge to the nervous system: generate actions to efficiently acquire rewards as necessary for survival) and subjective well-being so that it is important for individual matters[1]. For example, self-evaluations / maintenance, life satisfaction and subjective well-being are often derived from social comparisons[1, 2]. Many previous studies[3-10] had also suggested that reward processing was strongly context dependent (e.g., social comparison), and social comparison theory[1, 11] predicted that satisfaction with outcomes depended on relative comparisons with other people. Recently, some functional magnetic resonance imaging (fMRI) studies found that the striatum (e.g., the caudate nucleus) and other brain areas (e.g., the thalamus and the medial prefrontal cortex) in humans exhibited a high degree of context sensitivity in reward processing[3, 7, 10]. Specifically, Fliessbach et al.[7] found that socialcomparison (relative level reward) affected blood oxygenation level - dependent responses in the ventral striatum even if subjects were not actively engaged in decision-making, and their results suggested that mere contextual information about the other person had an immediate impact on reward-related brain processes. Although fMRI studies provided many important results to explore the brain mechanism of reward processing under social comparison, the time course of cortical activation could not be studied with precision. Recently, Qiu et al.[12] used a simple number estimation task and selected three relative reward levels (Self : Other = 1:2, Disadvantageous inequity; Self : Other = 1:1, Equity; Self : Other = 2:1, Advantageous inequity) as experimental conditions[7, 10, 12]. They found that both Disadvantageous andAdvantageous Inequity elicited a more negative ERP deflection (N350-550) than did Equity between 350 and 550 ms. The N350-550 might reflect monitoring and controlling reward prediction error. Then, Disadvantageous and Advantageous Inequity both elicited a more negative component (LNC) than did Equity between 550-750 ms, and the generator of LNC was localized near in the caudate nucleus, which might be related to reward processing under social comparison. By recording and analyzing ERPs elicited by different social comparison conditions, ERP data allow for more precise examinations of the electrophysiological correlates of the impact of social comparisons on the neural substrates of reward processing. However, there were still some shortcomings in Qiu et al.’s study. First, they devised a simple social comparison which included only two persons (one subject and one pseudo subject). Thus, there were two unambiguous reward feedback conditions, including Disadvantageous Inequity condition (worse off than some) and Advantageous Inequity (better off than many). In fact, Peoples need to compare themselves to many other peoples (three or more peoples) in their daily living. We also know that there is a famous saying, “worse off than some, better off than many”. Obviously, person comparison might be complex and pervasive in social judgment and human decision making. Second, they did not explore whether social comparison affected the spatiotemporal cortical activation patterns of subjective well-being(in present study subjective well-being was defind as satisfied with the payments when compare with others). As Fliessbach et al.[7] said, “The question of whether social comparison affects individuals’ subjective well-being, and thus behavior, is of fundamental importance, with far-reaching implications for the positive and normative predictions of economic theories.”. Thus, it is important for us to investigate the neural basis of how comparisons with other people influence feelings and behavior deeply.Therefore, based on previous study[12], high-density (64 channels) ERP recording was used to determine whether complex social comparison affected the spatiotemporal cortical activation patterns of reward processing and subjective well-being. We still used a simple number estimation task, similar to that used by Fliessbach et al.[7]. However, we devised a complex social comparison condition, in which each subject was told that he/she would perform a simple number estimation task with the other two subjects in the next laboratory simultaneously. Specifically, four social comparison stimulus categories (four relative reward levels/self reward related to other subjects’) were mainly prepared: Self:He:She = 1:2:4 (Disadvantageous Inequity); Self:He:She = 1:1:1 (Equity); Self:He:She = 4:2:1 (Advantageous Inequity); Self:He:She = 2:1:4 (Ambiguous Comparison). Our previous study had found that subjects were usually satisfied with Advantageous inequity and Equity feedbacks, and not satisfied with Disadvantageous Inequity feedback[12]. In the present study, we were interested in whether or not subjects would be satisfied with Ambiguous Comparison feedback, and tried to investigate the neural basis and brain areas related to reward processing of Ambiguous Comparison condition (“worse off than some, better off than many”). Based on previous studies[3, 5, 7, 10, 12] and social comparison theory[1, 11], we hypothesized that Disadvantageous Inequity, Advantageous Inequity and Ambiguous Comparison would elicit a more negative ERP deflection[e.g., error-related negativity (ERN)] than did Equity. Then, Ambiguous Comparison, Disadvantageous and Advantageous Inequity would elicit a greater negativity than did Equity, similar to our previous finding[12]. In fact, emotion effects on LPC have been found frequently[13-16]. For example, Inaba et al.[13] found that negative valence substantially increased episodic retrieval memory, and such increased recollection reflected the greater magnitude of the LPC. As well as, Schirmer et al.[16] found negative words elicited a larger late positivity than positive words. In the present study, according to social comparison theory, in most cases, subjects tended to experience more positive affect after exposure to others who were worse off (i.e., Advantageous inequity), and to experience more negative when compared with others who were better off (i.e., Disadvantageous Inequity)[1, 2, 17, 18]. Thus, we also anticipated that Disadvantageous Inequity would elicit a late more positive ERP component (LPC) than did Advantageous Inequity, Ambiguous Comparison and Equity in the late processing of reward feedback. The anatomic specificity data of fMRI mapping obtained from previous studies and the time resolution of ERP recordings would enable the characterization of the functional roles of specific brain areas in the context-dependent reward processing.
2. Mothod
2.1. Subjects
- As paid volunteers, 22 healthy undergraduate students (average age 21.6, range 19-24, ten men, and twelve women) from Southwest University (Chongqing) in China participated in the study. We had obtained appropriate ethics committee approval for the research, and all subjects gave written informed consent. All subjects were right-handed, had no history of current or past neurological or psychiatric illness, and had normal or corrected-to-normal vision.
2.2. Procedure
- Before the experiment began, each subject was told that he/she would perform a simple number estimation task with the other two subjects in the next laboratory simultaneously [one experimenter explained our experimental task for all three subjects (one is subject, and the other two are our experimental assistants) each time]. In fact, the two subjects were pseudo subjects and their performance and reward feedback were predetermined. That is, there was a high possibility (95%) of making a correct judgment for the pseudo subject when our subject was correct. Each subject was also told that he/she could earn additional money (e.g., 30 Yuan) which depended on his/her performance related to the other subject’s in each trial. An amount of money which he/she received at the end of experiment included the additional money and the primary show-up fee. The time course of a trial was illustrated in Figure 1. First, the fixation point appeared with 0.3 s duration at the center of the screen. Subsequently, subjects saw a screen with a varying number (10 to 50) of black dots for 1.5 s. Then, a number (e.g., 24) was presented. Subjects were required to judge whether the number of black dots was lower or higher than the number 24, and to rest their right index and right middle finger on the keys “1” and “2” of a keyboard. They need to press “1” if they thought that it was higher than the number 24 and to press “2” if they thought that it was lower than the number 24. After a correct response feedback (0.3 s) and a short delay (0.2-1 s), a reward feedback screen informed the subject about his and the other two subject’s performance and the respective monetary rewards (1.6 s). Finally, they were required to make a satisfaction judgment, and to press “1” if they thought that they were satisfied with the payments and to press “2” if not. The next trial started after a time interval of 1-1.5 s.
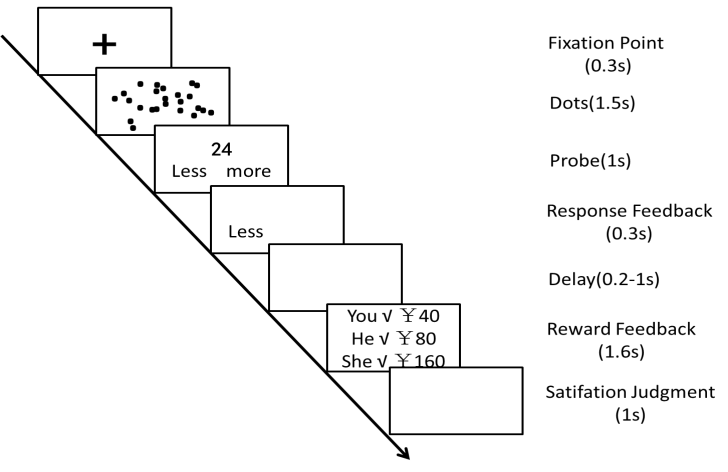 | Figure 1. Time course of a trial[revised on the figure 1 in Fliessbach et al.’s study, 2007] |
2.3. Stimuli
- The stimuli of reward feedback comprised 400 pairs of one subject’s and the other two pseudo subjects’ monetary rewards with respective performance. The size of stimulus was Song Ti No.20, and stimulus angle is 2.5o (horizontal) ×2.2o (vertical). The stimuli were displayed in the center of a 17-inch screen. Specifically, these stimuli included four main categories according to subjects’ performance. That is, reward feedback conditions were as follows: When all subjects were incorrect, they received nothing. When only one of the subjects was correct, this subject received some money while the other two subjects were not rewarded. When two of the subjects were correct, the subjects received some money while the other subject was not rewarded. When all subjects were correct, one of four possible conditions was randomly selected: Self:He:She=1:2:4 (20:40:80, 28:56:112, 40:80:160, 48:96:192. Disadvantageous inequity condition); Self:He:She = 1:1:1 (20:20:20, 28:28:28, 40:40:40, 48:48:48. Equity condition); Self:He:She = 4:2:1 (20:10:5, 28:14:7, 40:20:20, 48:24:12. Advantageous inequity condition) and Self:Self:He = 2:1:4 (10:20:40, 14:28:56, 20:40:80, 24:48:96. Ambiguous comparison condition).
2.4. ERP Recording and Analysis
- Brain electrical activity was recorded from 64 scalp sites using tin electrodes mounted in an elastic cap (Brain Product), with the average reference electrode on the left and right mastoids and a ground electrode on the medial frontal aspect. The vertical electrooculograms (EOGs) were recorded supra- and infra-orbitally at the left eye. The horizontal EOG was recorded from the left versus right orbital rim. All interelectrode impedance was maintained below 5 kΩ. The EEG and EOG were amplified using a 0.05-80 Hz bandpass and continuously sampled at 500 Hz/channel for offline analysis. Eye movement artifacts (blinks and eye movements) were rejected offline. Trials with EOG artifacts (mean EOG voltage exceeding ± 80 V) and those contaminated with artifacts due to amplifier clipping, bursts of electromyographic activity, orpeak-to-peak deflection exceeding ± 80 V were excluded from averaging.We analyzed the ERP elicited by Disadvantageous inequity, Advantageous inequity and Equity conditions. The averaged epoch for ERP was 1800 ms, including 1600 ms poststimulus and 200 ms prestimulus. As observed in the grand averaged waveforms (see Fig. 2), the ERPs elicited by Disadvantageous Inequity, Advantageous Inequity, Equity and Ambiguous Comparison conditions were clearly distinct from each other. The difference waves were obtained by subtracting the averaged ERP of Equity from the averaged ERPs of Disadvantageous Inequity and Advantageous Inequity, and all these differences were prominent over the frontal, central and occipital scalp regions. Thus, the following 5 electrode sites were selected for statistical analyses: Fz, FCz, Cz, CPz and Pz. The analyses of variance (ANOVA) factors were stimulus type (Disadvantageous Inequity, Equity, Advantageous Inequity and Ambiguous Comparison) and electrode site. For all analyses, p-value was corrected for deviations according to Greenhouse Geisser.
2.5. Dipole Source Analysis
- Brain Electrical Source Analysis program (BESA, Version, 5.0, Software) was used to perform dipole source analysis. For dipole source analysis, the 4-shell ellipsoidal head model was used. In order to explore and increase the precision of source location, principal component analysis (PCA) was employed in the ERPs difference waves of Disadvantageous inequity minus Advantageous inequity, Disadvantageous inequity minus Equity, Advantageous inequity minus Equity (64 channels). When the dipole points were determined, the software automatically determined the dipoles location. Source locations are described in Talairach–Tournoux coordinates. To evaluate the solutions, the residual variance (RV), which provides an estimate of the amount of ERP power not explained by the seeded dipoles, was calculated by comparing the squared total error to the squared data (data power).
3. Results
3.1. Behavioral Performance
- In the simple number estimation task, the mean accuracy rate was 95.8% ± 0.4% and the mean response time (RT) was 457 ± 26 ms. For Disadvantageous Inequity, Equity, Advantageous Inequity and Ambiguous Comparison conditions, mean trials of each condition were 67 ± 5, 66 ± 5, 67 ± 5 and 61 ± 7. Satisfaction judgment rates for Disadvantageous Inequity, Equity, Advantageous Inequity and Ambiguous Comparison conditions were 29.8 ± 8.6%, 88.7 ± 4.4%, 88.9 ± 5.2% and 60.0 ± 8.2%, respectively. Repeated measures ANOVA for satisfaction judgment rates showed that there was a significant effect of stimulus type[F (3, 63) = 23.21, P < 0.05]. Pairwise comparison showed that subjects were more satisfied with Advantageous Inequity and Equity than Ambiguous Comparison (P < 0.05) and Disadvantageous Inequity (P < 0.05), and subjects were also more satisfied with Ambiguous Comparison than Disadvantageous Inequity (P < 0.05). To some extent, these results might be consistent with the social comparison theory (e.g., Festinger, 1954; Wills, 1981) which predicted that subject’s satisfaction with outcomes (e.g., reward) depended on relative comparisons with other people.Mean RTs of satisfaction judgment for Disadvantageous Inequity, Equity, Advantageous Inequity and Ambiguous Comparison conditions were 437.4 ± 107 ms, 422.4 ± 104 ms, 424.3 ± 99 ms and 464.9 ± 134 ms. Repeated measures ANOVA for RTs showed that the main effect of stimulus type was significant[F (3, 63) = 3.50, P < 0.05]. Pairwise comparison showed that RTs for Equity was much faster than RTs for Ambiguous Comparison (P < 0.05) and Disadvantageous Inequity (P < 0.05), and RTs for Ambiguous Comparison was slower than RTs for Disadvantageous inequity (P < 0.05), Equity (P < 0.05), Advantageous Inequity (P < 0.05).
3.2. Electrophysiological Scalp Data
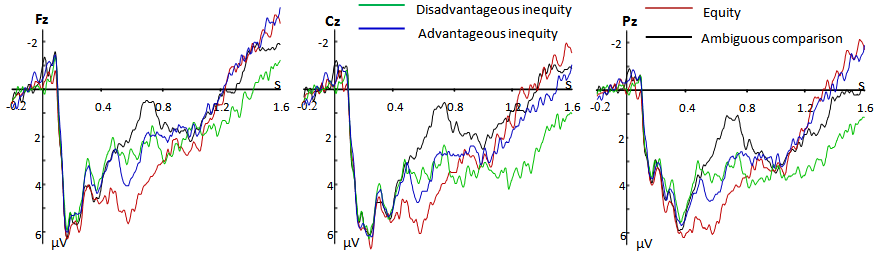 | Figure 2. Grand average ERPs at Fz, Cz and Pz for the four conditions |
- The grand-average ERPs waveforms (Fig. 2) showed the following spatiotemporal distribution for the ERP data. After onset of the stimuli, the N1 and the P2 were elicited by Disadvantageous Inequity, Equity and Advantageous Inequity. Then, Disadvantageous Inequity, Equity and Advantageous Inequity elicited a late positivity component after 350 ms. Amplitudes and latencies of the N1 and the P2 and mean amplitudes in the time windows of 350-550, 550-750 and 1200-1400 ms were analyzed using 2-way repeated measures ANOVAs.The results of the ANOVAs showed that there were no main effects of stimulus type for the amplitude and the latency of the N1. We also didn’t find main effects of stimulus type for both the amplitude and the latency of the P2. Then, there was a main effect of stimulus type in the time window of 350-550 ms, F (3, 63) = 3.22, P < 0.05. Pairwise comparisons showed that Disadvantageous, Advantageous Inequity and Ambiguous Comparison all elicited a more negative deflection (N350-550) than did Equity (P < 0.05). In addition, the interaction stimulus type and electrode site was not significant[F (12, 252) = 1.53, P > 0.05]. Between 550-750 ms, there was a main effect of stimulus type, F (3, 63) = 7.24, P < 0.01. Pairwise comparison showed that a more negativity in Ambiguous Comparison (LNC1), Disadvantageous (LNC2) and Advantageous Inequity (LNC3) as compared to Equity, and Ambiguous Comparison elicited a more negativity (N550-750) than didAdvantageous Inequity and Disadvantageous Inequity. There was also a main effect of electrode site in the time window of 550-750 ms, F (4, 84) = 9.31, P < 0.05. However, the interaction stimulus type and electrode site was not significant, F (12, 252) = 1.25, P > 0.05. Lastly, there was also a main effect of stimulus type in the time window of 1200-1400 ms, F (3, 63) = 3.82, P < 0.05. Pairwise comparisons showed that Disadvantageous Inequity elicited a late more positive ERP component (LPC) than did Advantageous Inequity (P < 0.05), Equity (P < 0.05) and Ambiguous Comparison (P < 0.05). The interaction stimulus type and electrode site was also not significant[F (12, 252) = 1.53, P > 0.05].
3.3. Dipole Source Analysis
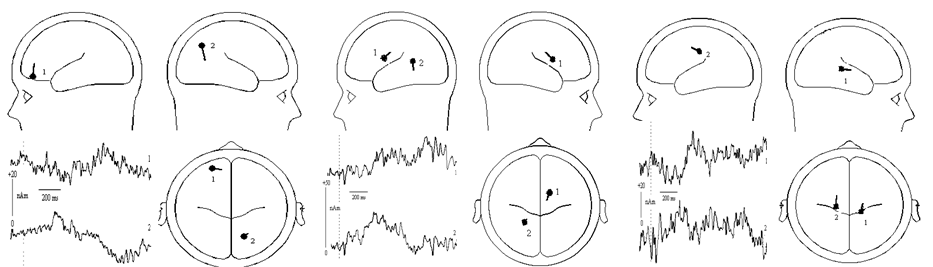
- Source analysis was performed on the three difference waves of Disadvantageous Inequity minus Equity and Ambiguous Comparison minus Disadvantageous Inequity. PCA were employed in the 350-550 ms, 550-750 ms and 1200-1400 ms time windows because there were main effects of stimulus type and the ERPs elicited by Disadvantageous Inequity, Equity, Advantageous Inequity and Ambiguous Comparison were clearly distinct from each other. We determined the number of dipoles on the basis of the results of PCA and our own scientific hypotheses. First, PCA was employed in the difference wave (Disadvantageous Inequity minus Equity) between 350-550 ms. PCA indicated that two components were needed to explain 86.5% and 5.5% of the variance in the data. Therefore, two dipoles were fitted with no restriction to the direction and location of dipoles. The result indicated that the first dipole was located near in the medial frontal/ anterior cingulate cortex (location according Talairach coordinates: x = -24.8, y = 50.0, z = -0.7; ACC, BA10/32), and the second located near the precuneus (x = 16.3, y = -60.0, z = 34.5; BA7). This model explained the data best and accounted for most of the variance with a RV of 16.0% and revealed maximal dipoles moment strength at about 475 ms (see Fig.3). PCA was also employed in the difference waves (Advantageous inequity minus Equity and Ambiguous Comparison minus Equity) between 350-550 ms, and we found there was a similar result. Second, as for the difference wave of Disadvantageous Inequity minus Equity between 550-750 ms, PCA indicated that two components were needed to explain 88.3% and 4.2% of the variance in the data. Therefore, two dipoles were fitted with no restriction to the direction and location of dipoles. The result indicated that the first dipole was located near in the left caudate nucleus (x = 12.7, y = 2.9, z = 22.1), and the second located near the posterior cingulate cortex (x = -22.0, y = -44.0, z = 12.9; PCC, BA30). This model explained the data best and accounted for most of the variance with a RV of 7.5% at the peak activity of these dipoles (see Fig.3). In addition, we also found that there was a similar result for the difference waves (LNC1 or LNC3).Third, PCA was employed in the difference wave (Ambiguous Comparison minus Disadvantageous Inequity) between 550-750 ms. PCA indicated that two components were needed to explain 76.7% and 12.6% of the variance in the data. Therefore, two dipoles were fitted with no restriction to the direction and location of dipoles. The result indicated that the first dipole was located near in the left caudate nucleus (x = 16.1, y = -29.5, z = 9.0), and the second located near the ACC (x = -18.6, y = -22.5, z = 37.9; BA24). This model explained the data best and accounted for most of the variance with a RV of 7.0% and revealed maximal dipoles moment strength at about 680 ms (see Fig.3). However, the dipole source analysis of the difference waves in the 1200-1400 ms time window didn’t indicate any effective generators. The validities of these models were tested through the following steps. First, the display of the residual maps in the time windows (350-550 ms and 550-750 ms) showed no further dipolar activity; second, no other dipoles could be fitted in the investigated time windows by comparing the solution with other plausible alternatives (e.g., bilaterally symmetric dipoles). These tests might suggest that the models explained the data in the best manner for the time windows.
4. Discussion
- In the present study, we used ERPs to explore the electrophysiological correlates of reward processing and subjective well-being in the complex social comparison context. Our results showed that Disadvantageous, Advantageous Inequity and Ambiguous Comparison all elicited a more negative ERP deflection (N350-550, LNC) than did Equity in the time windows of 350-550 and 550-750 ms. Then, Disadvantageous Inequity elicited a LPC than did Equity, Advantageous Inequity and Ambiguous Comparison conditions between 1200-1400 ms. Moreover, the medial frontal/ACC and the caudate nucleus might be related to reward processing under social comparison. We would discuss the implication of these findings in our study.First, observing from the grand-average ERPs, Disadvantageous, Advantageous Inequity and Ambiguous Comparison conditions all elicited a more negative ERP deflection (N350-550) than did Equity between 350-550 ms, similar to our previous findings[12]. We used the simple number estimation task to make subjects generate expectations of feedback based on their and the other subject’s performance. According to our subjects’ subjective reports and behavioral data, we found that they usually expected their rewards should be the same as the other two subjects’ when they were all correct[10]. Then, a difference would be detected under Disadvantageous Inequity (Not satisfaction with inequitable outcomes), Advantageous Inequity (Satisfaction with inequitable outcomes) and Ambiguous Comparison (Satisfaction with inequitable outcomes) when subjects compared the expected feedback to the actual feedback. That is, we thought that the N350-550 might be similar to the N400[19], the feedback-related negativity[20] or the error-related negativity[21]. Holroyd et al.[8] found that the ERN might be elicited by a reward prediction error (e.g., the difference between expectations and actual outcomes), such that unpredicted non-rewards elicited the largest ERNs, and suggested that the ERN might be generated by the impact of reinforcement learning signals carried by the mesencephalic dopamine system (MDS) on the ACC[22, 23]. Moreover, we indeed found that the generator of the N350-550 was localized near in the medial frontal/ACC. Previous studies[24, 25] had indicated that the medial frontal/ACC might act as part of a more general performance monitoring system (whether feedback was better or worse than expected), and improve performance due to its role in cognitive control and action monitoring [26-28]. Therefore, we thought that the N350-550 in our study might reflect monitoring and controlling reward prediction error (a difference between expectations and actual outcomes) during reward processing under social comparison which consistent with our hypotheses. Second, Ambiguous Comparison, Disadvantageous and Advantageous Inequity all elicited a more ERP deflection (LNC) than did Equity between 550-750 ms, similar to our previous findings[12]. The generator of LNC (e.g., Disadvantageous inequity minus Equity) was localized in the left caudate nucleus, which might be related to reward processing under social comparison. The LNC was commonly characterized as slow waveform, which showed modulation in late time-course and reflected higher-order cognitive processes[29-31]. Some previous studies also indicated that the LNC might be involved in working memory, such as temporarily storing, manipulating, and maintaining information[32, 33]. Moreover, previous studies had found that the striatum (e.g., the caudate) of humans exhibited a high degree of context dependency in reward processing[3-6, 8, 9, 14, 34]. Fliessbach et al.[7] also indicated that the ventral striatum responses to a variation in the comparison subject’s payment indicated that people did not evaluate objects solely by their absolute value but that social comparison played a substantial role in evaluation of reward. Therefore, the LNC might reflect evaluating for the rewards under social comparison after detection of reward prediction error in our study which consistent with previous studies..More important and interesting, we found that Ambiguous Comparison elicited a greater negativity (N550-750) than did Disadvantageous and Advantageous Inequity, and the generators of N550-750 were localized in the caudate nucleus and the ACC, which might be related to reward processing and cognitive conflict controlling under social comparison. Our behavioral data showed that it was difficult for the subjects to make a satisfaction judgment under Ambiguous Comparison than Disadvantageous inequity and Advantageous inequity conditions (RTs for Ambiguous Comparison was slower than RTs for Disadvantageous and Advantageous Inequity). We believed that subjects might spend much more cognitive resources for reward processing in the social comparison context under Ambiguous Comparison (worse off than some, better off than many) compared to Disadvantageous (worse off than some) and Advantageous Inequity (better off than many) conditions because they need to compare themselves to many other different peoples. Many previous studies had found that the ACC might play an important role in implementing the processes underlying adjustments of performance control[4, 35, 36]. Botvinick et al.[26]indicated that ACC activation can be explained by the single function of the detection of conflict and puts forth the hypothesis that conflict might serve as an index of the demand for mental effort. Under Ambiguous Comparison condition, subject’s reward outcome (Self:He:She = 2:1:4) was worse off than one but better off than the other. They were difficult to make an advantageous/disadvantageous and satisfaction/ dissatisfaction judgment, and this most likely contributed to the ACC activities in our study. Based on these previous findings and our results, we suggested that these brain regions (e.g., the caudate nucleus and the ACC) probably recruited processes of elaborated evaluating for the rewards under social comparison after detection of reward prediction error, and Ambiguous Comparison (“worse off than some, better off than many”) might be more similar to human’s decision making in daily living. To some extent, our results also proved the view that brain activation to the same rewards differed depending on relative comparisons with other people[3, 6, 37, 38] and on the set of possible outcomes from which the actual reward was chosen[10, 39].Third, Disadvantageous Inequity elicited a late more positive ERP component (LPC) than did Advantageous Inequity, Equity and Ambiguous Comparison conditions between 1200-1400 ms. Some previous studies had indicated that negative/positive slow waves in the ERP are correlated with rehearsal/retention operations in working memory[40-42]. So, we thought that the LPC might reflect emotional experience induced by reward outcomes under different social comparsion condition. In fact, emotion effects on LPC have been found frequently[13-16]. For example, Inaba et al.[13] found that negative valence substantially increased episodic retrieval memory, and such increased recollection reflected the greater magnitude of the LPC. As well as, Schirmer et al.[16] found negative words elicited a larger late positivity than positive words. In the present study, according to social comparison theory, in most cases, subjects tended to experience more positive affect after exposure to others who were worse off (i.e., Advantageous inequity), and to experience more negative when compared with others who were better off (i.e., Disadvantageous Inequity)[1, 2, 17, 18]. Our behavioral data also showed that subjects tended to make dissatisfaction judgments under Disadvantageous inequity condition, and to make satisfaction judgments under Advantageous Inequity, Inequity and Ambiguous Comparison conditions. Thus, our results indicated that social comparison indeed affected individual well-being, which was of central importance for understanding behavior in social environment. In this study, the LPC might reflect strong unpleasant emotional experience induced by Disadvantageous Inequity compared to Advantageous Inequity, Equity and Ambiguous Comparison conditions which consistent with our hypotheses. In a word, the present study deeply investigated the electrophysiological correlates of the impact of social comparisons (worse off than some, better off than many) on the neural substrates of reward processing. Results showed that these ERP components (the N350-550, the LNC and the LPC) and these brain areas (the medial frontal/ACC and the caudate nucleus) might be related to reward prediction error, evaluating for the rewards under social comparison. Based on previous studies[7, 12], this present findings supported the view that mere contextual information (reward feedback outcomes) about other persons might have an immediate impact on individuals’ motivation-related brain processes. Of course, due to inherent limitations of source localization, the brain areas implied by source localization were only tentative. Regarding the involvement of brain regions in response to Ambiguous Comparison, Disadvantageous Inequity, Equity and Advantageous Inequity, the current results only provided a model, rather than empirical data. In the future, further studies should be done using both ERPs and fMRI to investigate spatiotemporal cortical activation patterns underlying the brain mechanism of context - dependent reward processing.
ACKNOWLEDGEMENTS
- This research was supported by the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 200806351002), the National Natural ScienceFoundation of China (30800293), the Key Discipline Fund of National 211 Project and the Key Laboratory of Human Development and Mental Health in Hubei (Central China Normal University) (200803).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML