-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Psychology and Behavioral Sciences
p-ISSN: 2163-1948 e-ISSN: 2163-1956
2012; 2(6): 263-273
doi: 10.5923/j.ijpbs.20120206.10
Modification of Serial Pattern Learning by Designer Tryptamine Exposure during Adolescence: Comparison with Damage to the Dorsal Hippocampus or Prefrontal Cortex
David M. Compton 1, Melissa C. Selinger 2, Eric Westman 1, Peter Otero 1
1Department of Psychology, Palm Beach Atlantic University, 901 S. Flagler Drive, West Palm Beach, FL 33401, USA
2Gregory School of Pharmacy, Palm Beach Atlantic University, 901 S. Flagler Drive, West Palm Beach, FL 33401, USA
Correspondence to: David M. Compton , Department of Psychology, Palm Beach Atlantic University, 901 S. Flagler Drive, West Palm Beach, FL 33401, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Foxy or Methoxy Foxy (5-MeO-DIPT) is one of a series of new “club drugs” that within the past decade has gained in popularity among recreational users as an alternative to MDMA (Ecstasy). Unlike MDMA, not much is known about the neurobiological consequences of 5-MeO-DIPT use. Little is known about the effects of either compound on learning in a nonspatial appetitive task. In the present study, adolescent rats were given repeated injections of 10 mg/kg of 5-MeO-DIPT, MDMA, or a corresponding volume of isotonic saline. In serial learning tasks, depending on task demands, there is a growing body of evidence suggesting that multiple memory systems play a critical role, with each system playing a more or less dominant role depending on the available stimuli and task demands. Therefore, for comparison purposes, the drug-treated rats were compared with that of hippocampus- or prefrontal cortex-lesioned rats. After adolescent drug exposure or lesions during adolescence, adult animals were trained All animals were trained for 30 days on a three-element, nonmonotonic pattern consisting of 21, 0, and 7 food pellets, respectively. Control rats were capable of distinguishing among the elements of the series, as indexed by running times. As expected, the tracking performance of the lesioned rats was impaired. Performance in both the 5-MeO-DIPT- and the MDMA-treated rats improved with training but after 30 days was not markedly different than the lesioned animals. The results are discussed in terms of measured alterations in serotonin activity in the forebrain and the consequences of compromised serotoninergic systems on the cognitive processes involved in appetitive serial learning tasks.
Keywords: 5-MeO-DIPT, Foxy, MDMA, Ecstasy, Serial Learning, Development, Hippocampus, Prefrontal Cortex
Cite this paper: David M. Compton , Melissa C. Selinger , Eric Westman , Peter Otero , "Modification of Serial Pattern Learning by Designer Tryptamine Exposure during Adolescence: Comparison with Damage to the Dorsal Hippocampus or Prefrontal Cortex", International Journal of Psychology and Behavioral Sciences, Vol. 2 No. 6, 2012, pp. 263-273. doi: 10.5923/j.ijpbs.20120206.10.
Article Outline
1. Introduction
- Like other mammalian species, rats are capable of tracking the elements of a stimulus series consisting of differing reinforcement quantities[see 1, for a review]. Although other methods have been employed[e.g., 2], demonstrations of serial learning in rats involves exposure to a three- to seven-element series consisting of differing numbers of food pellets. Anticipatory responding is inferred through the demonstration of different running times to a goal box as a function of the given reinforcement quantity.While a number of theoretical models have been developed[3], the bulk of the evidence supports the existence of two associative mechanisms that can explain rodent serial-pattern learning - (1) the development of stimulus-stimulus associations[4,5] or (2) the ordinal position of each element of the stimulus series comes to function as a differential cue[6,7]. In a variation of the latter theoretical view, a series of reinforcement events is converted to a spatial array[8].A consensus has emerged that a hippocampus-dependent memory system, normally labelled as declarative memory, is critical for learning the multiple relationships among stimuli[9,10]. This system is considered essential in order for the organism to learn information about and then flexibly utilize information about relationships between multiple external cues and events[9,10]. A second dorsal striatum-dependent system has been described as necessary for the formation of reinforced stimulus-response associations[10-13]. Both systems may be essential for serial learning but their relative importance is driven by task requirements[1].Serial pattern learning involves flexible responding in the face of anticipated changes in the environment. A large body of research has implicated the prefrontal cortex (PFC) in many aspects of cognition as well as executive processes[14]. Specifically, the PFC appears to be an essential component in learning and memory, decision making, and cognitive control over behaviour[15-18]. Last, past research has consistently indicated the involvement of the PFC in behavioural flexibility[18-20].A variety of learning and memory impairments following 3,4-methylenedioxymethamphetamine (MDMA) exposure have been reported in both nonhuman animal studies[21-26] as well as research involving human subjects[27-30]. In humans, MDMA-induced impairments involve a variety of cognitive deficits including alterations in working memory or prospective memory, as well as disruptions in executive functioning[27-30].Many of the MDMA-induced deficits have been linked to observed reductions in brain serotonin (5-HT) levels[31] and this effect has been observed across species (see 32, for a review). Of particular interest here, 5-HT reductions are seen in a number of regions involved in different types of learning and memory, and include such critical brain regions as the hippocampus, the dorsal striatum, and the prefrontal cortices[31]. Further, the effect is observed in adult rats[21] as well as rats exposed when they are young[24-26]. Of considerable import, alterations in 5-HT function have been reported to continue long after the MDMA exposure period[32-36].As is the case for many other tryptaminergic drugs, 5-Methoxy-N,N-di(iso)propyltryptamine hydrochloride (5-MeO-DIPT; FOXY) has become popular among recreational users. FOXY has properties very similar to that of other tryptaminergic hallucinogens[37]. As a consequence, recreational users of MDMA and other similar compounds have experimented with this drug. However, since it is similar to other tryptamine compounds of abuse, there have been reports of the negative consequences associated with FOXY use as a recreational drug[e.g., 38,39]. As reports of its use accumulated, in the United States FOXY was classified as a Schedule I drug[40]. Although some recent work has elucidated some of the effects of this compound[38-43], unlike MDMA, our knowledge of the consequences associated with the use of FOXY on the behaviour and neurobiology of mammalian systems remains limited.Adolescence in rats includes the period from the 21st day following birth (postnatal day; PND) until PND 60[44,45]. In addition, adolescence can be subdivided into mid adolescence (PND 34 to 46) and late adolescence (PND 46 to 59). According to Tirelli et al.[45], these two developmental periods are analogous to periadolescence and late adolescence/early adulthood. Rodent models of adolescence models are useful for comparative assessments and for extrapolation to humans[46]. Specifically, the use of adolescent animals allows for a valuable experimental framework for testing the developmental effects associated with drugs of abuse at various time points in biological and cognitive development.Of the published investigations, only a select few[e.g., 41,43] have specifically examined the effects that FOXY may have on cognition or explored the long-term consequences associated with exposure at different points in brain development. Somewhat more is known about MDMA. However, no one has explored the effects of these compounds on a nonspatial cognitive learning task such as serial pattern learning. Unfortunately, the popularity of these drugs remains high and, as a consequence, the possible risks to development in vulnerable adolescents could be seen as an emerging societal health problem. Therefore, the present study was conducted to provide further elucidation into the consequences of developmental exposure to MDMA and FOXY.
2. Method
2.1. Subjects
- The subjects consisted of 33 male Long-Evans rats (Charles River, Boston, MA) 35 days of age at the beginning of the study. After arrival in the vivarium at 21 days of age, the animals were allowed to acclimate to the facility and were randomly assigned to one of five groups hereafter designated as follows: a control group (CON) consisting of n = 8 rats, two groups of drug-treated animals exposed to (±)-3,4-methylenedioxymethamphetamine (MDMA, n = 7) or 5-MeO-DIPT (FOXY, n = 7) and, two lesion groups receiving bilateral lesions of the dorsal hippocampus (HIP, n = 6) or the prefrontal cortex (PFC, n = 5). Four of the eight control rats received sham lesions where the electrode was lowered into the target area but no current was passed. The rats were individually housed and maintained on a 12-hr light/12-hr dark cycle, with all testing conducted during the light phase. With the exception of serial learning training, the animals were maintained with ad lib access to food and water. The research protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Palm Beach Atlantic University and the animals were treated in accordance with the principles of animal care outlined in the Guide for the Care and Use of Laboratory Animals[47].
2.2. Apparatus
- The apparatus consisted of a wooden enclosed runway 185 cm long, 10 cm wide, and 14 cm high, with each section covered with hinged Plexiglas. The start and goal boxes (20 & 35 cm in length) were separated from the runway by two manually operated guillotine doors. The start box was painted flat white, the main runway flat grey, and the goal box flat black. Raising a guillotine door located between the start box and the main runway activated one digital timer (Lafayette, Model 20225) which was stopped when the rat interrupted a photobeam located 15 cm within the goal box. The goal box contained a removable ceramic dish with walls high enough to obscure the food reinforcement until the rat was physically inside the goal box. All food reinforcement consisted of a predetermined number of 0.045-g Noyes food pellets. To minimize the presence of odor cues that might have influenced performance[48], the floor of the apparatus was swabbed with a wet sponge and dried with a paper towel before presentation of each element of the series.
2.3. Behavioural Procedure
- Four days before pretraining began, each rats was weighed and reduced to 85% of their free-feeding weight. This 15% reduction was maintained throughout the experiment by feeding a daily maintenance ration (about 14 g) of Mazuri Rodent Chow. Water was available ad lib.After the rats' weights were stabilized at the targeted 15% reduction, a two-day pretraining program began. During this period, individual rats were hand tamed for a two-minute period, followed by a five-minute period where the rats were free to explore the apparatus. Both guillotine doors were elevated during this phase. Last, each rat was permitted to consume 21 food pellets located in the food dish located in the goal box.Behavioural training began when the rats were approximately 100 days old. Following the two days of pretraining, the experimental training began and continued for 30 days. The rats were transported in their home cages in squads of three animals with the training order randomized daily. All rats received two daily trials consisting of a three-element ordered sequence of 21, 0, and 7 food pellets (Noyes: 0.045-g). Within each three-animal squad, all rats completed trial one before any rat experienced trial two. The intertrial interval was approximately 15 minutes with within-trial inter-element intervals of approximately 1 minute. Once the rat successfully traversed the runway, it was confined in the goal box until all of the food pellets were consumed or for 30 seconds on 0-pellet elements. When a rat did not reach the goal box within 60 seconds, it was gently pushed toward the goal box, confined there until all food pellets, if any, were consumed, and a 60 second running time was recorded. After consumption of all of the pellets and following each element of the series, the rat was returned to its holding cage while the experimenter swabbed the floor of the apparatus, baited the food-well, and reset the timer and guillotine doors. After the completion of both daily trials, the rats were returned to the rat colony, where they received their maintenance ration of Mazuri Lab Chow not less than 90 minutes later.
2.4. Surgical Procedures
- All surgeries were performed under anesthesia consisting of pretreatment with .25 mg/kg atropine followed 10 minutes later by 40 mg/kg of Nembutal. Behavioral pretraining began no less than 10 days following a postoperative recovery period.Following the appropriate plane of anesthesia, lesions of the dorsal hippocampus were created as follows. Bilateral openings were made in the cranium with a trephine. Electrode placement was determined on the basis of stereotaxic coordinates determined from the atlas of Paxinos and Watson[49]. Following placement of a stainless steel electrode insulated except at the tip, bilateral electrolytic lesions were created in the appropriate site. The coordinates calculated from bregma and current parameters were AP = -3.8 mm, ML = ±1.5 mm, DV = -3.3 mm, 2 mA for 20 seconds and AP = -3.8 mm. ML = ±2.5 mm. DV = -3.4 mm for 20 seconds. Prefrontal cortex ablations involved removal of areas of the cortex that correspond to those described as the medial precentral, anterior cingulate, and prelimbic cortex as defined by Krettek and Price[50]. Last, as noted earlier, four animals were placed in the stereotaxic device and prepared similarly for lesions, but did not receive lesions (i.e., shams).
2.5. Histological Analysis & Biochemical Analysis
- For animals who received lesions, following data collection the subjects were deeply anesthetized (Nembutual, 50 mg/kg) and perfused intracardially with 40 cm3 of isotonic saline, followed by a 10% formalin solution. The brains were removed and stored in a 30% sucrose-100% formalin mixture for 48 hours before being frozen. All brains were sectioned in the coronal plane at 60-µm intervals, mounted on slides, and stained with cresyl violet acetate.HIP lesions were examined under microscopic magnification against stereotaxic atlas templates using the atlas of Paxinos and Watson[49]. Viewed from a dorsal perspective, all cortically ablated brains were photographed. Subsequent analysis of cortical surface lesions proceeded with a dot grid method[51]. The dot grid method permits placement of a digital grid containing 256 dots per square inch over a Lashley diagram with the accompanied ablation. The number of dots contained within the area of the traced cortical lesion is counted, thus providing an estimate of the amount of cortical surface area destroyed.Approximately, five weeks after the completion of training on the serial learning task, the animals exposed to MDMA or FOXY were euthanized. Serotonin levels were assessed in a manner described elsewhere[42]. Briefly, we determined 5-HT levels in the hippocampus, striatum, and the prefrontal cortex using high performance liquid chromatography (HPLC; a Waters Model 600 with electrochemical detection). Procedures were consistent with that outlined by Chapin, Lookingland, and Moore[52] with modification. The system used a Waters C18 reverse phase analytical column (3.9 X 300 mm; 4 µm). Concentrations in the amounts of 0.04% sodium octyl sulfate, 0.1 mM disodiumethylenediamine-tetraacetate, 0.05 M sodium phosphate were dissolved in laboratory-grade H20 using 0.03 M citric acid as a buffer. The aqueous portion of the mobile phase was held within pH levels between 2.7 and 2.9. The mobile phase consisted of 20% methanol and 80% aqueous phase. 5-HT levels were calculated and reported as ng/g tissue.
|
2.6. Data Analysis
- In order to normalize the data, the running times were transformed using the reciprocal (X= 1/X) data transformation[53]. The data were collapsed into three blocks consisting of days 1through 6, 7 through 18, and 19 through 30, respectively and were analyzed, using a three-way ANOVA: 3 (groups) x 3 (blocks) x 4 (elements). We treated groups as a between-subjects factor, whereas blocks and elements were treated as within-subject factors. We used TukeyHSD tests to analyze the within-group differences in running times to the series elements.
3. Results
3.1. Histological Analysis
- Visual examination of the HIP-lesioned animals revealed the following (see Figure 1). Substantial damage to the overlying cortex, corpus callosum, and cingulum was seen in all animals as was considerable damage to the fimbria. All animals had minor damage to the laterodorsal area of the thalamus and the stria terminalis. One animal received minor damage to the anterodorsal region of the thalamus. In addition, extra-hippocampal structures with minor damage included the dorsal lateral geniculate nucleus (one animal), the anterior pretectal nucleus (one animal), and the paraventricular nucleus (one animal).Examination of the PFC lesions indicated that they generally involved the area from the frontal pole to the genu of the corpus callosum. With the exception of one rat, the tissue along the medial wall of the medial walls of the saggital sulcus, including the majority of the cingulate gyrus, was undamaged. Conversely, some involvement of the anterior cingulate gyrus was found in all five animals. Using the dot grid method described previously, suggested that when the lesions were considered from the dorsal perspective that they were uniform in size (M = 0.21, SD = 0.013).
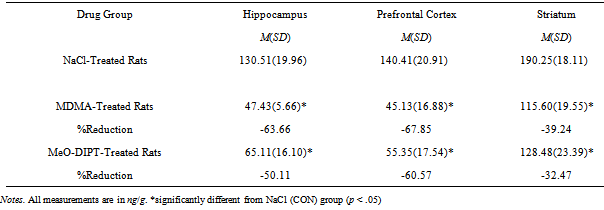
3.2. Neurochemical Analysis of Brain 5-HT Levels
- The mean levels of 5-HT in the hippocampus, prefrontal cortex, and striatum are reported in Table 1. The 5-HT levels differed as a function of drug exposure in each measured area (hippocampus, F(2, 19) = 60.94, p < .001; prefrontal cortex, F(2, 19) = 59.68, p <.001; striatum, F(2, 19) = 29.29, p < .001. Post hoc examination of these results using TukeyHSD tests revealed the following differences among groups. When compared to the CON (NaCl-treated) animals, an examination of the 5-HT levels in the hippocampus indicated significant reductions in 5-HT (63.66% & 50.11%) in both the MDMA and FOXY drug groups. However, when the two drug groups were compared, the 5-HT levels were comparable (i.e., p >.05). Reductions in prefrontal 5-HT levels were observed in both the MDMA-treated rats (67.85%) and the FOXY-treated rats (60.57%). However, the difference in 5-HT levels in both drug-treated groups was nonsignificant. Last, when compared to control animals, significant reductions in striatal 5-HT were also observed (39.24% for MDMA & 32.47% for the FOXY rats). The pattern of differences between the CON group and two drug groups but not the two drug groups as measured 5-HT levels in these target areas is consistent with previous work[42].
3.3. Behavioural
- The goals of the present investigation were to determine (1) if prior exposure of MDMA or FOXY disrupted the ability of rodents to learn a nonmonotonic serial pattern and (2) compare the results to that of hippocampal or PFC lesioned rats. Therefore, learning performance was considered at points within the 30 days of training. To do this, the data were collapsed into three blocks consisting of days 1 through 6, 7 through 18, and 19 through 30. In order to meet the assumptions associated with the analysis of variance (ANOVA), all running times were transformed using the reciprocal transformation. The serial learning performance of each group collapsed into the three training period blocks is presented in Figure 2. A one between (drug or lesion groups), two-between (blocks & elements) ANOVA revealed the following. A main effect of group was found, F(4, 28) = 6.71, p < .01, indicating that running times differed across the training period. As the main effect of blocks, F(2, 56) = 64.25, p < .05, suggests, the animals improved across the training period and running times differed, F(2, 56) = 74.57, p < .05, as a function of run within the three-element series. The group X blocks and group x elements interactions were both significant as well (Fs(8, 56) = 3.30 & 8.96, ps < .01, respectively). Here, the results suggest that group differences emerged both across the three blocks associated with the early, middle, and late training periods as well as within individual elements of the series.
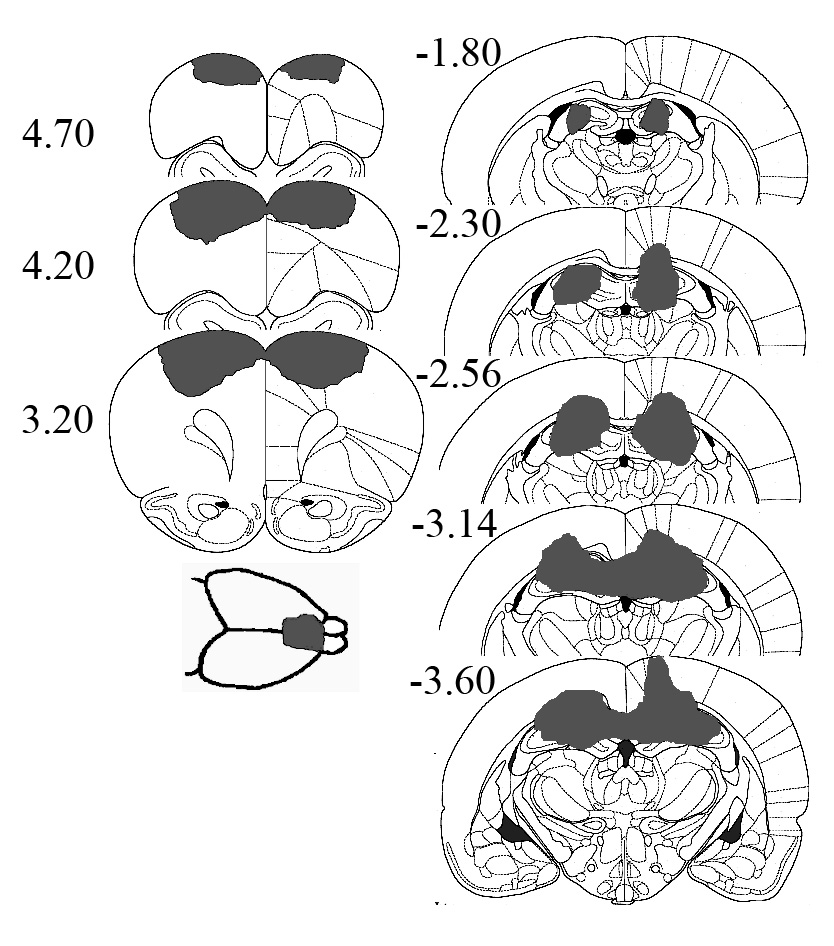 | Figure 1. Representative PFC and HIP lesions. Numbers are relative to bregma as defined by Paxinos and Watson[49] |
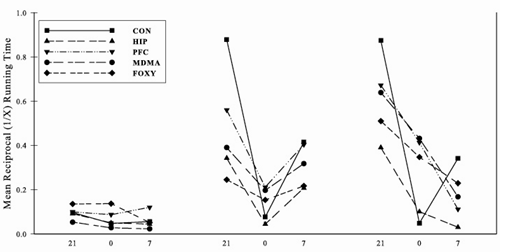 | Figure 2. Mean reciprocal running times in three blocks consisting of days 1 through 6, 7 through 18, and 19 through 30, respectively |
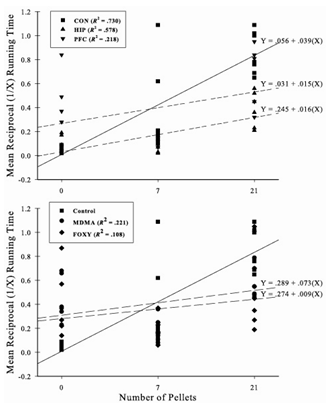 | Figure 3. Mean reciprocal running times in three blocks consisting of days 1 through 6, 7 through 18, and 19 through 30, respectively |
4. Discussion
- The purpose of the present experiment was to examine the impact of adolescent exposure of MDMA and FOXY on the acquisition of appetitive serial learning task. A second goal was to compare the performance of the drug-treated rats with that of HIP- and PFC-lesioned animals, two regions implicated in serial pattern learning[1] and processes such as rule-learning and response flexibility[57]. Exposure to either drug during adolescence produced a marked learning impairment in the serial learning task in (drug-free) adulthood. The deficits were as severe as that of the brain lesioned groups, with the performance of the drug-treated animals all but indistinguishable from that of the PFC animals. Only the CON animals demonstrated accurate tracking performance. In related work[42], no differences in performance on the rotating balance beam or in levels of general activity have been found suggesting that the deficits were not simply a result of motor deficits.There is a growing body of evidence suggesting that multiple brain areas are involved in sequential learning and memory[58]. In humans, this is supported from work with drug-treated[59] or brain damaged individuals on serial reaction time tasks[60,61]. The work of Nissen and colleagues supported the proposal that serial learning involves activity within both declarative and nondeclarative (procedural) memory systems[59,60; see also, 62]. Individuals with Alzheimer’s disease or experimental participants treated with the acetylcholine antagonist scopolamine improved across trials with a 10-element sequence but had no explicit recognition for the learning experience[59,60]. As such, the results suggested that serial learning relies on a nondeclarative memory system. Conversely, Huntington’s disease patients, with a compromised nondeclarative memory system that includes but is not limited to the basal ganglia showed no improvement. Last, much like Huntington’s patients, individuals with Parkinson’s disease have sequence learning deficits that are independent of more general impairments in motor performance[58,63,64].As Muller & Fountain[58] noted, the available evidence suggests that rats may rely on at least three cognitive processes in serial pattern learning. The use of memory for items in a series may involve any combination of (1) the processing of external discriminative cues, (2) counting time encoding processes for each position of the elements of the series and/or, abstraction of a rule or rules for encoding an internal representation of the structure of the pattern[58].Consistent with the earlier discussion, in rats mastery of an appetitive serial-pattern learning task is influenced by at least two dissociable neural systems. First, animals are capable of learning the serial pattern using a declarative memory system that includes the hippocampus, incorporating stimulus-stimulus and rule learning[1,58,65]. If this system is compromised or if the salience of stimulus elements is skewed, then a nondeclarative memory system that includes the dorsal striatum will permit the rat to learn the series[1]. In fact, the nondeclarative system appears to drive the formation of reinforced S-R responses, with a third system that includes the amygdala appearing to contribute to the affective aspects of the experience[66-69). Which neural system is dominant is largely determined by the stimuli available to predict a given trial outcome and the complexity of the specific serial pattern[1]. The prefrontal cortex is one of a number of critical brain regions that are involved in the ability to respond in an effective manner when confronted with changing contingencies between a stimulus and response[15,70]. In serial pattern learning, such flexibility is critical as contingencies (trials) change. Indeed, there is evidence that depletion of prefrontal/orbitofrontal 5-HT is highly correlated with the perseverative impairments[71,72]. Further, experimental manipulations of 5-HT levels in the cerebral cortex leading to lower 5-HT levels are associated with an enduring increase in response impulsivity[62]. In the present experiment, determination of whether the observed deficits were a result of cognitive flexibility or other processes, or both is not readily determined. As noted earlier, rodent serial learning involves multiple brain structures and multiple learning strategies that differ depending on task demands[1,58]. Last, it has been observed that genetic variations in the monoaminergic transporter protein SERT impact cognitive flexibility[73]. Reductions in SERT binding following MDMA exposure are considered indicative of serotonergic axonal damage[74].In the present study, FOXY-treated rats were impaired relative to CON animals with the impairment approaching that of MDMA-treated rats. Although generally the effects of the former do not appear to be as severe as the latter[43,75], neither appear to diminish with age[42]. In a related study, we compared adolescent exposure of FOXY with MDMA and periodically tested the animals across the lifespan with the preliminary results indicating that the deficits largely remain throughout the lifespan[76]. In one recent investigation[43], rats treated with FOXY during postnatal day 11–20 were impaired relative to control animals in spatial learning but not tests of spatial memory or path integration. However, in related work with adult rats[77], a path integration deficit was observed. Of relevance here, the authors suggest that the difference is possibly a reflection of hippocampal development[c.f., 78] that occurs during the exposure period used in their study.When considering factors such as exposure to drugs of abuse, the period of exposure during biological development is a relevant variable[43]. For example, in one study of 5-HT turnover in the nucleus accumbens of rats[79], levels of 5-HT turnover in the nucleus accumbens were four times lower in adolescent rats than prepubescent rats (PND 10 to 15) or adult rats. Further, in rodents, 5-HT2A receptor in the cortex is at its peak just before the onset of adolescence, followed by a gradual decline to adult levels[80]. Thus, the timing of exposure of each of the compounds could have a variety of effects that differ markedly depending on when exposure takes place during neural development, what other drugs are taken concurrently, and the length of exposure.Data from the neurochemical assessment of 5-HT revealed substantial reduction in 5-HT levels measured in the prefrontal cortex, hippocampus, and the striatum. This result is largely in accord with previous research with rats[41-43,75,76] and mice[81]. As noted above, the timing of exposure is important, with some reports suggesting more persistent 5-HT reductions if the drug exposure period includes early adolescence rather than during a later developmental period[81]. In addition, multiple doses of MDMA can produce measurable 5-HT toxicity in periods greater than 100 days following exposure[82]. Nonetheless, the available evidence is mixed as there are some reports of an absence in the reduction of 5-HT levels following adolescent exposure[83] and some species differences (rats vs. mice) have been observed[82,84,85]. One additional caveat concerning the present results is noteworthy. While significant reductions in 5-HT levels were detected after exposure to both MDMA and FOXY, linking 5-HT levels with that of neurotoxicity is still an area of debate[74]. This is true even if measured using different methods, including the methods employed here and elsewhere[43,74] and radioligand binding in SERT studies[86,87]. While the issue cannot be settled here, excellent discussions of the issues can be found in the literature[88-90]. At any rate, regardless of whether the drug-induced deficits were related to axonal damage or another process, on a functional level the animals were impaired when tested as adults, long after adolescent exposure and is consistent with previous reports[42,43].The present study lends additional support to the suggestion that there is a developmental period of vulnerability to the effects of both MDMA and FOXY. Perusal of recommendations on the internet[e.g., 91,92] suggest that concurrent use of selective serotonin reuptake inhibitors with MDMA can ameliorate or even prevent the adverse side effects or even damage caused by MDMA[74]. In the present study as well as reports by others[42,43], exposure to MDMA and FOXY appear to produce lasting consequences. Since other neurotransmitter systems (e.g., dopamine, norepinephrine) may be compromised by the use of these compounds as well[43,74,75,93,94], it is imperative in future research to examine the behavioural consequences in youth who use such drugs for recreational purposes. By doing so, more effective ameliorative and therapeutic strategies can be developed.
ACKNOWLEDGEMENTS
- This research was sponsored in part by a grant from the Palm Beach Atlantic University Faculty Research Committee to David Compton. The authors would like to thank N. Hernandez and K. Dietrich for their expert technical assistance.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML