M. A Hamada 1, E A. Gomaa 1, N A. El-Shishtawi 2
1Department of Chemistry,Faculty of Science, Mansoura University , Mansoura, 35516, Egypt
2Department of Physics, Faculty of Science, Mansoura University , Mansoura, 35516, Egypt
Correspondence to: E A. Gomaa , Department of Chemistry,Faculty of Science, Mansoura University , Mansoura, 35516, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
The optical and mechanical properties of 10% by weight for PVA (Polyvinyl alcohol) in presence of different CoCl2 concentrations in absence and presence of 44 % by weight ethanol – water solvents were estimated at 298.15 K. The evaluated optical parameters like molar refraction (RM),specific refractivity(ε) and molecular polarizability (α) were compared and discussed with that of mechanical tension parameters like elastic modulus and Shear modulus.
Keywords:
Optical Properties, Mechanical Properties, Polyvinyl Alcohol, Cobalt Chloride, Ethanol Water Solvent
Cite this paper:
M. A Hamada , E A. Gomaa , N A. El-Shishtawi , "Optomechanical Properties of 10% PVA (Polyvinylalcohol) in Presence of CoCl2 and 44% Ethanol Water Compositions", International Journal of Optoelectronic Engineering, Vol. 2 No. 1, 2012, pp. 1-3. doi: 10.5923/j.ijoe.20120201.01.
1. Introduction
Polymer solutions like PVA solutions are important in applied electrochemistry due to their applications in electrodes,especially when doped with transition metals (1,2) Transition metals plus polymer solutions improve the conducting properties and play important role in forming thin films (3). Conductivity and refractive index measurements explain the ion - ion, ion – solvent Interactions and the association of ions in different media (4,5). Estimation of the optical and mechanical properties of polymers in absence and presence of metals are very important to give relations between the optomechanical characterizations (6). The aim of this work is to evaluate the optical and mechanical properties of PVA in presence of CoCl2 and solutions in presence and absence of both PVA (polyvinyl alcohol) and 44% by weight ethanol – water mixtures together or separately.
2. Experimental
Cobalt chloride from Merck Co. and polyvinyl alcohol (PVA), M.W 17,000, water soluble polymer from Arondate laborations were provided. The ethanol was BDH supplement and water used was secondly distilled one. 5 ml of bidistilled water were put in test tubes, then different concentrations of solid CoCl2 were added and dissolved. Also another solutions were prepared by adding different con- centrations of CoCl2 to 44% by weight ethanol-water solventsin absence and presence of 10% (by weight) PVA. The solutions were left for more than one day in water thermostat of the type (Polyscience 8105, USA) at 298.15 K. The densities were measured by taking 1ml of the prepared solutions and put in specific gravity bottle (1 ml capacity) and weight them by the use of Mettler Toledo USA four digital balance with accuracy of plus, minus 0.001 gram. The densities have been used for evaluating the solvated radii (7). The refractive indices of the different solutions used here in this work were measured using a refractometer of the type ATAGO-3T (No 5250) at 298.15 K.
3. Results and Discussions
From densities of CoCl2 aqueous solutions in absence and presenceof 10% by weight PVA, the molar volumes were evaluated by dividing the molecular weight of CoCl2 by densities, and the evaluated volumes (V) are represented in Table 1.From the experimental refractive indices (n), the molar refraction (RM) were calcu44% by weight latedBy applying (8) eq.1.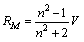 | (1) |
Where V is the measured molar volumes.The evaluated RM values are listed also in Table 1. The specific refractivities of the isotropic dielectric (ε ) were calculated by using de Vries equation (9,10) as seen in eq.2.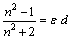 | (2) |
Where d are the densities of the solutions. The mean values of the molecular dipole polarizability(α) i.e the dipole moment induced by electric field were calculated from Lorenz-Lorenz formula (11) following equation (3)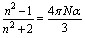 | (3) |
The number of molecular chains (N),the molduli of elasticity E and Shear modulus G were calculated by using equations (4), (5) and (6) after Fouda and Gomaa and Mognashi (10-12).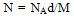 | (4) |
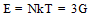 | (5) |
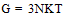 | (6) |
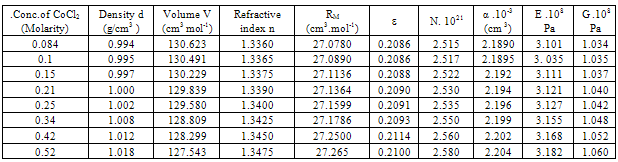
Where k is Boltzman,s constant , T the absolute temperature , NA is the Avogadro,s number and M is the molecular weight of CoCl2Table 1. Densities (d),molar volume (V),refractive index (n), molar refraction (RM), specific refractivity ( ε ) number of chain molecules ( N ).polarizability (α ).elastic modulus (E) and Shear modulus (G) for CoCl2 in aqueous solutions at 298.15K
 |
| |
|
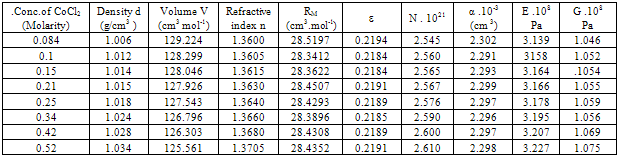
Table 2. Densities (d),molar volume (V),refractive index (n), molar refraction (RM), specific refractivity ( ε ) number of chain molecules ( N ).polarizability (α ).elastic modulus (E) and Shear modulus (G) for CoCl2 in 44 % by weight ethanol water solutions at 298.15K
 |
| |
|
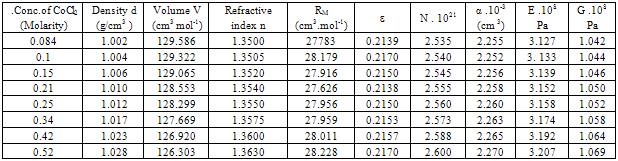
Table 3. Densities (d),molar volume (V),refractive index (n), molar refraction (RM), specific refractivity ( ε ) number of chain molecules ( N ).polarizability (α ).elastic modulus (E) and Shear modulus (G) for 10% PVA + CoCl2 in aqueous solutions at 298.15K
 |
| |
|
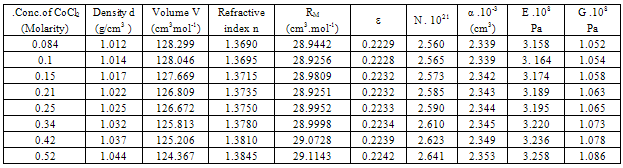
Table 4. Densities (d),molar volume (V),refractive index (n), molar refraction (RM), specific refractivity (ε) number of chain molecules (N).polarizability (α).elastic modulus (E) and Shear modulus (G) for 10% PVA + CoCl2 in 44 % mixed ethanol water solvents at 298.15K
 |
| |
|
All the measured and evaluated data for CoCl2 aqueous solutions (Table 1), CoCl2 in mixed ethanol-water solvents (Table2) and 10% polyvinylalcohol + CoCl2 in absence of 44 % ethanol-water mixed solvents (Table 3) and in presence of 44 % mixed ethanol-water solvents (Table 4), are evaluated at 298.15 K. In comparing the four given tables we can conclude that both the optical and mechanical properties of CoCl2 are increased by using mixed 44% ethanol-water solvents and 10 % PVA. CoCl2 + 10% PVA + 44% ethanol-water solutions show the biggest optical and mechanical properties from all the used media.The organic solvents increase the salvation parameters (13-15) affecting the optical and mechanical properties.Therefore the optical and mechanical properties of PVA solutions can easily be improved by the addition CoCl2 and 44 % ethanol-water solutions.
4. Conclusions
The optomechanical properties of PVA increase in presence of CoCl2+44% ethanol by weight due to the increase in molar refraction (RM), specific refractivity (ε), molecular polarizability (α), elastic modulus (E) and Shear modulus (G ).
References
| [1] | D.H .Han, , H.J Lee, and M.S Park,.,.Electrochimica Acta,50, 3085, 2005 |
| [2] | U.N. Dash,...Mahapata.J .R. and Lal ,B.Journal of Molecular liquids.124,13 , 2006 |
| [3] | E.G Lyon, (Ed), Electroactive Polymer Electrochemistry, Part 1. Plenum Press.New York, 1992 |
| [4] | U.N. Dash,..and M.R Patnaik,.Chem.Envirom.Res.,5(1-4), 259, 1996 |
| [5] | U.N .Dash, and B . K Mohanty, Indian J.Chem., 35A (1996) 983 |
| [6] | A.A Hamza,. I.M Fouda,. M.A Kabeel.., A Seisi and. F.M El-Sharkawy,J.Appl.Polm.Sci. 65,2031, 1997 |
| [7] | E.A Gomaa, Proc.Kon.Neder.Ak.Van Weten,91B, 363, 1988 |
| [8] | J.B .Hasted,”Aqueous Dielectric”, Chapman and Hall, London, 1973 |
| [9] | H. De Vries,., Z. Colloid.Polym.Sci.,257,226 ,1979 |
| [10] | I.M Fouda, and A.H Oraby , , Polymer Testing,208,25 -833, 2001 |
| [11] | N.A.El-Shistawi,M.A.Hamada and E.A.Gomaa.,Chemistry, 18, 146 , 2009 |
| [12] | E.R ,Mognashi, and L..M.Laboranti, ,,J.Chem.Soc.,Faraday Trans.,92, 3369 , 1996 |
| [13] | E.A.Gomaa, B. M.Al-Jahdali, American Journal of Fluid Dynamics,1(1)4-8 , 2011 |
| [14] | N.A.El-Shishtawi, M.A. Hamada and e.A .Gomaa, Physical Chemistry,1(1)14-16,2011 |
| [15] | E.A.Gomaa,Analele Univ.din Bucuresti-Chimie, 19, (1), 45-48 , 2010 |

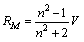
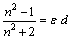
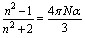
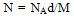
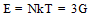
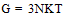
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML


