-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Metallurgical Engineering
p-ISSN: 2167-700X e-ISSN: 2167-7018
2020; 8(1): 1-6
doi:10.5923/j.ijmee.20200801.01

Review on the Mechanism of L360 Steel in H2S-CO2-Cl- Environment under Elemental Sulfur Deposition
Muhammad Baba Pali, Jiling Li
School of Chemistry and Chemical Engineering, Xi’an Shiyou University, Xi’an, Shanxi, P.R. China
Correspondence to: Muhammad Baba Pali, School of Chemistry and Chemical Engineering, Xi’an Shiyou University, Xi’an, Shanxi, P.R. China.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Deterioration due to corrosion in terms of economic aspects includes repair and conservation costs, loss of materials, deterioration to equipment, a decline in efficiency, and loss of functional or productive life. This review summarizes the recent research and development on the mechanism of L360 steel in H2S-CO2-Cl- environment under elemental sulfur deposition, status at domestic and oversea, influencing factors and characterization methods were reviewed and discussed. The information presented in this review is vital for diverse industrial fields, primarily for guiding the safe development of high sulfur-bearing oil and gas.
Keywords: Mechanism, L360 steel, Elemental Sulfur, Predicament
Cite this paper: Muhammad Baba Pali, Jiling Li, Review on the Mechanism of L360 Steel in H2S-CO2-Cl- Environment under Elemental Sulfur Deposition, International Journal of Metallurgical Engineering, Vol. 8 No. 1, 2020, pp. 1-6. doi: 10.5923/j.ijmee.20200801.01.
Article Outline
1. Inroduction
- This review brings together the information from the studies relevant to production fields that elemental sulfur deposition takes place with sour surroundings, basically in greater concentration of H2S level reservoirs. In an aqueous state, exposure of solid sulfur with mild steel can cause disastrous corrosion predicaments. Elemental sulfur deposition due to numerous chemical and physical purposes unveil the possibility of dissociation of hydrogen sulfide or polysulfide to yield elemental sulfur. Therefore, dissociated elemental sulfur starts to deposit in the appearance of little particles and sticks to the inner surface of the pipeline. Elemental sulfur constantly functions as a cathode to the steel pipe because of their unequal potential. As soon as free water or conductive condensates pass over the deposited elemental sulfur particle, an active electrochemical cell will be set up. When this materialized, a precipitated localized corrosion will take place and establish a pit [1-3]. The development of this pit will be extremely improbable to be managed even if corrosion control is administered into the system [4]. Consequently, discharge of flammable and toxic gases will be anticipated in such an area. Downstream of a point of pressure depletion which is also connected with temperature reduction is the most common site for sulfur deposition in natural gas transmission pipelines whereby localized corrosion is anticipated in such an area [4]. Greater number of researchers documented the gravimetric method to expect the elemental sulfur corrosion for natural gas pipeline use [5]. It is reviewed that the study of corrosion has advanced from macro to micro-scope with the support of microscopic research, which will aid further to unveil the corrosion mechanism in essence and progressively especially the pitting corrosion mechanism, which is a matter of close concern in the field of corrosion.
2. Status at Domestic and Oversea
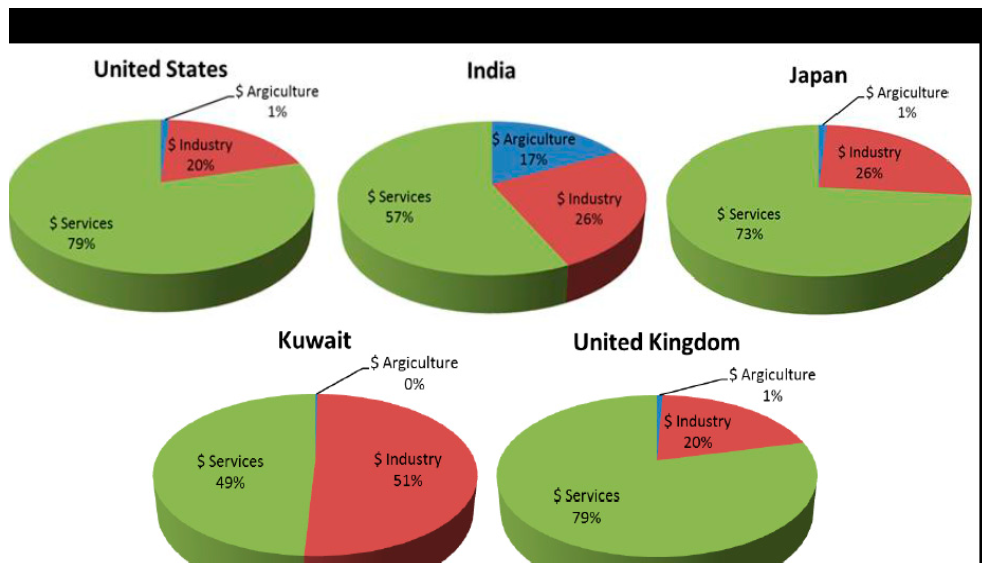
- This paper review and discusses the prevalent corrosion predicaments relating to foreign countries such as Hunter valley acid pipeline (H2S 14%), (CO2 3.3%) which was corroded and perforated by elemental sulfur deposition after 13 months of operation, and the apparent concave point was ¼ circumferential along the low position of the pipeline. The corrosion site is 300m long, which is isolated pitting corrosion because of the low air flow rate in the pipeline and the accumulation of elemental sulfur and formation water with high Cl- content also caused corrosion perforation in pipelines [6]. In 2014, the Gulf of Mexico South Coast pipeline also experienced corrosion perforation because of elemental sulfur deposition, ensuing in (bbl) leaks of 50 barrels of crude oil, the accident investigation revealed that the perforation was located underneath the pipeline and was isolated pitting corrosion [7]. With an estimated GDP of 1261 billion dollars in Mexico, approximately 3% is considered to represent their cost of corrosion predicaments [8]. Guangzhou Gas Field is the first large bulk marine gas field in China with high sulfur content, due to the low flow rate of acid gas gathering pipeline, the element sulfur is effortless to gather and deposit, and the deposited sulfur is mostly in the form of S8 [9]. The corrosion predicament of gathering pipeline caused by sulfur deposition is becoming more and more prominent [10], which led to oil/gas and H2S leakage [11]. Moreover, corrosion is mainly dense in the direct synergy between metal and elemental sulfur, which is manifested as local corrosion, mainly pitting corrosion [12,13]. It is reviewed in this paper the study of five heterogeneous sectors relative to their economic section as shown in Figure 1. According to the reports of these five sectors, it is revealed that there is a homogeneous corrosion costs monotonous to developed industries and services economies in the regions of United Kingdom, Japan and the United States, whereas Kuwait and India had a considerable participation from the oil industry and agricultural economies, respectively [14]. Consequently, substantial amount of the consequences caused by corrosion can be saved through adequate corrosion prevention, surveillance, and applying safety quality and practices in these categories ranging from 15%–35% [14].
2.1. Influencing Factors of Elemental Sulfur Corrosion
- 1. Temperature and PressureTemperature and pressure in steels have been studied in order to understand corrosion reaction rates, reaction mechanism, microstructure and morphology. It is therefore reviewed in this paper that the composition and morphology of corrosion products are straightly influenced by temperature and partial pressure, which in turn promoted to the alteration of corrosion rate and occurrence of localized corrosion [16]. Consequently, the localized corrosion established is generally determined on the time exposure to temperature. It is proposed that the corrosion rate is mainly restrained by the appearance of iron sulfide scale [17]. Increase in sulfidation rate is generated by an increase in the temperature and H2S concentration. It is unveiled that when temperature is higher than 80°C, sulfur would react with water, resulting in the serious acidification of the corrosive solution [18]. Fang [19] discordant that when temperature goes beyond 80°C, sulfur could take place hydrolysis reaction clearly and then water medium acidulated. Simultaneously, sulfur is a strong oxidant, sulfur agglomeration on steel surface readily takes place disproportionation reaction to generate H2S gas under a certain temperature and medium conditions [20]. 2. Hydrogen Sulfide (H2S) Numerous studies were conducted over the years, it is reviewed in this paper that H2S is among the influencing factors of corrosion in the oil and gas industry. The study of corrosion under elemental sulfur deposition in S-water, S-NaCl, H2S-CO2 and S-H2S systems reveal that these factors are always coupled together to jointly influence the corrosion dynamic behavior. Simultaneously, the progressive transformation and interaction of numerous mitigation steps in corrosion system also promoted the complexity of the system [21]. Furthermore, the corrosiveness of the environment with high H2S and CO2 can be progressively enhanced by the presence of sulfur and even the nickel-based alloys may experience adverse pitting corrosion under the deposited sulfur, which will further the menace of corrosion incapacity of tubing, casting and pipelines [22-23]. Localized corrosion with a pitting rate of 55mm/a, is usually ensued by failure events of gathering pipeline, pipeline perforation, oil and gas and H2S exposure. In the oil and gas industries, localized corrosion precipitate to waste of resources and environmental pollution, then consequently results in endangering of human health and life safety. This means that for high sulfur oil and gas fields, local corrosion caused by elemental sulfur deposition makes the oil and gas gathering and transportation pipeline system frail in safety and dependability. Local corrosion under elemental sulfur deposition, particularly pitting corrosion has become a tough predicament in corrosion control of oil and gas gathering and transportation pipeline in high sulfur oil and gas fields [23].3. Carbon dioxide (CO2):Numerous studies have been accomplished in order to be cognizant with the corrosion of steel in a CO2 medium, and there is a comparative harmony on the mechanisms, which are repeatedly interconnected to the emergence of an iron carbonate scale. CO2 for numerous years has been determined as one of the main agent of corrosion in the oil and gas industry [24]. CO2 can be dissolved in aqueous condition to form acidic, corrosive solution [25]. CO2 is hydrated to form carbonic acid when dissolved in water, which is corrosive to steel. The detriments rely on temperature, oxygen content, pH, CO2 partial pressure and solution chemistry greater than alloy composition and hydrodynamic of the set up [26-33]. Generally, proliferation in CO2 partial pressure proliferates the steel uniform corrosion rate [27-31]. Depending on the corrosion rate, CO2 corrosion is conventionally managed by addition of inhibitors with determined concentrations [32-33] or by using stainless steels which are more resistant to corrosion such as alloys [34].4. Chloride ion (Cl-):Hitherto in a research conducted by Valery (2011) on the effect of chloride ion concentration on the corrosion rate of carbon steel in solutions saturated with H2S (at room temperature) which is shown in Figure 2. It is reviewed that intensification in the chloride ion concentration, within the limits from 0 to 3.6% NaCl, proliferates the corrosion rate exponentially, and it is propounded that the chloride ions restrain the initiation of the sulfide films as well as proliferating the conductivity of the solution, thereby, enhanced corrosion rate [35]. The figure below was adopted to depict the effect of NaCl concentration on corrosion rate.
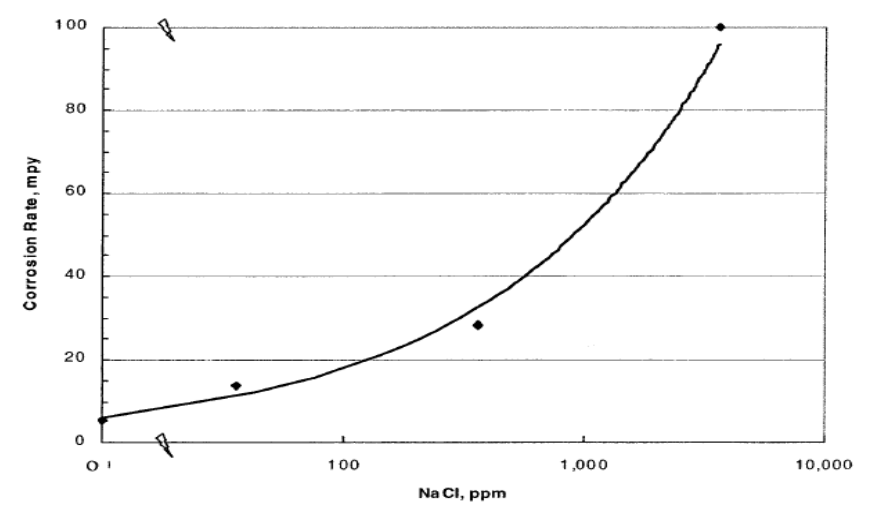 | Figure 2. Effect of NaCl concentration on the corrosion rate of carbon steel in a H2O- H2S -NaCl system (after Foroulis) [35]. Picture Source, Adopted from Valery (36) |
2.2. Characterization Methods
- Over the years petroleum industries have experienced corrosion predicaments due to elemental sulfur deposition which represents a significant financial loss in the industry. It is paramount to determine the causes of these problems not only to assign proper responsibilities but to avoid the recurrences of such predicaments in the future. Therefore, the main characterization methods used in this review study for the determination and analysis of corrosion includes Scanning Electron Microscopy (SEM), X-ray Diffraction (XRD) and Mössbauer Spectroscopy, Electron Probe Micro-analysis (EPMA), Transmission Electron Microscopy (TEM), Energy Dispersive X-ray Spectroscopy (EDS) and Electrochemical Impedance Spectroscopy (EIS).1) Scanning Electron Microscopy (SEM) SEM is used to study and generate a variety of signals at the surface of a specimen to depict details, including the structure and composition of the corrosion product film formed. Previously, Khaksar et al. (2017) conducted a research work to analyze the surface morphology and corrosion product using Scanning Electron Microscopy (SEM). It is revealed on each sample that the SEM micrograph of the corrosion product layers formed on the surfaces was covered with elemental sulfur at pH 5 under 10, 20 and 30 h immersion time in thioacetamide solution [44]. In addition, it is possible for the observation and analysis of pitting growth using the scanning electron microscopy technique this is because in a research work conducted using scanning electron microscopy to show the SEM micrographs show that for the pit growth in the two test water with added SO42-, thick salt films were formed inside the pits or covered the pit mouths. Cracks in the salt films and gaps between the salt films and the base metal were also clearly observed [45].2) X-ray Diffraction (XRD) and Mössbauer Spectroscopy:With XRD and Mössbauer Spectroscopy, these two techniques are mostly used for the analysis and identification of corrosion products established in the pipeline and sediments dragged during pigging procedures [46-48]. It is also achievable to identify inorganic compounds and determine them both quantitatively and qualitatively. Previously, Mössbauer technique was numerously used to suggest reaction mechanism developed during corrosion process and results were obtainable [49]. Gayosso et al. (2016) conducted a research work to analyze the sediment collected from variant pipelines belonging to the same region, the result revealed the oxide and sulphide contents in the corrosion products obtained from the sediments dragged during pigging activities in many gas and crude oil pipelines [50]. Similarly, using XRD analysis in most of the sediments obtained from the gas pipeline revealed elemental sulfur [50].3) Electron Probe Micro-analysis (EPMA): Electron Probe Micro-analysis is a technique that is widely used in the analysis of element distribution of corrosion products. Liu et al. (2014), conducted a research work to analyze element distribution across corrosion product film, the result revealed that S, Fe, and O were formed after 96 hours immersions. It is depicted by the element distribution formed that the inner layer is rich in iron and the outer layer is rich in sulfur, which probably indicates that the inner layer consists of mackinawite and the outer layer consists of pyrrhotite and troilite. [51]4) Transmission Electron Microscopy (TEM):Corrosion has remained a major predicament in the oil and gas industry which drew the attention of many researchers on how to detect and determine the cause of such prevalent issues. Transmission Electron Microscopy (TEM), is a technique which has been used for decades to analyzed corrosion product. Liu et al. (2014), did a research work to analyze the micrographs with electron diffraction pattern of the iron sulfide formed on a steel after 2h immersion time at 90°C in 1.61 MPa H2S solution, the analysis revealed mackinawite, troilite and amorphous FeS. [51] 5) Energy Dispersive X-ray Spectroscopy (EDS):With energy dispersive x-ray spectroscopy, it is very feasible to conduct elemental analysis on cross-sections of the pits found on steels, and the elemental mapping can be carried out on the entire cross-section, while point analysis can be conducted on the pit wall or the product inside the pit. Research conducted for the analysis of pitting corrosion using EDS revealed that the pitting corrosion was formed on the entire specimens and a very significant size of pits was observed on the steel from the SO42- free simulated AVT water solely with 100 ppm Cl-. Research performed to analyze the chemical elements of corrosion products in 13Cr stainless steel at variant temperature using EDS whereby the analysis of the findings revealed that the corrosion scale mainly consist of C, S, Cr and Fe [45].6) Electrochemical Impedance Spectroscopy (EIS):Electrochemical impedance spectroscopy (EIS) is a powerful technique for characterizing a variety of electrochemical systems and for determining the contributions of electrodes or electrolytic processes as well as the corrosion rate. EIS technique has been used to study molecular structure and reactivity, the difference of corrosion behavior caused by local environmental change under elemental sulfur deposition. In the aspect of theoretical analysis, EIS will be used to analyze thermodynamic and kinetic parameters of impurity structure, the effect of impurity ions on the quantum properties of corrosion product films using the principle of corrosion electrochemistry. Thus, characterization is possible particularly in the linear polarization and potentiodynamic polarization scan which can give much information on the pitting susceptibility and passivity, cathodic behavior of an electrochemical system as well as the corrosion rate. In another research work, linear polarization of electrochemical measurement was used to examine the low corrosion rates formed [52]. Thus, Ayagou et al. conducted a research work on electrochemical impedance spectroscopy of iron corrosion in H2S solutions. The study revealed a variant behavior, with a 45° tail at low-frequency, interpreted by diffusion limitation which iron sulfide was formed. The impedance measurements conducted at brief immersion period and then replicated after 23 hours, and a significant high-frequency semi-circle was observed and revealed it was associated with a decrease in corrosion rate [53].
3. Conclusions
- After analyzing and understanding all the above literature, it can be seen that sulfur played a major role in the corrosion predicaments affecting oil and gas industries. Therefore, further corrosion research will provide information regarding the fundamental kinetics and mechanisms of the corrosion process because corrosion predicaments amount to a greater part of the total costs for oil and gas producing companies every year, because of corrosion escalation, investigating in oilfield application worldwide is very paramount. Moreover, greater theoretical and practical knowledge in essence to deeply explore the pitting corrosion system and the dynamic behavior for guiding the safe development of high sulfur-bearing oil and gas can help in preventing numerous potential predicaments that can cause debilitating issues including negative social impacts, water resource, environmental pollution and loss of life.
ACKNOWLEDGEMNTS
- This work was supported by National Natural Science Foundation of China (51974245, 21808182), Scientific Research Program of Shaanxi Provincial Education Department (18JS088) and Natural Science Basic Research Plan in Shaanxi Province of China (2019JM-506).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML