-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Metallurgical Engineering
p-ISSN: 2167-700X e-ISSN: 2167-7018
2017; 6(2): 31-35
doi:10.5923/j.ijmee.20170602.01

Feasibility Study of Dephosphorization of Slag Generated from Basic Oxygen Furnace of an Integrated Steel Plant
Vikash Kumar, Somnath Kumar, Jwala Prasad, K. K. Keshari, Somnath Ghosh, Asit K. Bhakat
Research & Development Centre for Iron & Steel, Steel Authority of India Limited, Ranchi, India
Correspondence to: Asit K. Bhakat, Research & Development Centre for Iron & Steel, Steel Authority of India Limited, Ranchi, India.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Basic oxygen furnace slag (BOF slag) is an unavoidable by-product of steelmaking process of basic oxygen furnace. The main chemical constituents of BOF slag are CaO, FeO, Al2O3, SiO2 and MgO. Due to its high metallic value (FeO: 16-20%) and lime content (CaO: 45-50%), it is possible to use in iron making and steelmaking process to replace lime and recover iron and CaO. But it also contains some P2O5 around 2 – 3% which is too high for recycling. The objective of this study is to investigate the removal of phosphorus by magnetic separation technique. The slag generally contains a Ca19Fe2(PO4)14 (phosphorus enriched phase) with FeO-CaO-SiO2 matrix phase. X-ray fluorescence (XRF), X-ray diffraction (XRD) and Scanning electron microscope (SEM) analysis were carried out for characterizing different property of BOF slag. The slag was then crushed and heated at several rates and dwell time up to 1500°C. Afterward it was cooled to 1400°C at the rate of 1°C/min with holding period of 240 min and then quenched with water. The cooled slag was then crushed, milled and separated by wet magnetic separation method. Since the magnetic properties differ for different phases, it is possible to separate each phase with the aid of magnetic separation. By applying magnetic field to the crushed slag sample, phosphorus lean and rich phases may be notably separated.
Keywords: Basic oxygen furnace slag, Dephosphorization, Wet magnetic separation, Characterization, Magnetic field
Cite this paper: Vikash Kumar, Somnath Kumar, Jwala Prasad, K. K. Keshari, Somnath Ghosh, Asit K. Bhakat, Feasibility Study of Dephosphorization of Slag Generated from Basic Oxygen Furnace of an Integrated Steel Plant, International Journal of Metallurgical Engineering, Vol. 6 No. 2, 2017, pp. 31-35. doi: 10.5923/j.ijmee.20170602.01.
Article Outline
1. Introduction
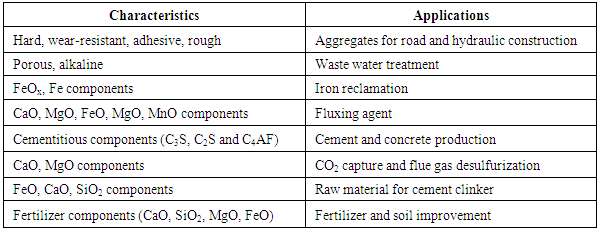
- Large quantities of steel slag are generated by the various steelmaking processes; of which basic oxygen furnace slag accounts larger fraction [1]. Steel and steel slag annual output of 2013 in India reached to 81.20 million tons and 11.36 million tons respectively. However, the current utilization rate of steel slag in India is only 20 % i.e. 2.27 million tons, far behind the developed countries like USA, Australia and European Countries etc. of which the rates have been 70, 70 and 80% respectively [2]. However, with steel production on the rise, slag production is also expected to increase manifold. In contrast with other nations, most slag produced in India, especially steel slag is discarded. However, this is increasingly becoming a problem due to paucity of land. These BOF slags are for recycled into two main ways; external reuse for road construction and hydraulic engineering, fertiliser etc and Internal recycling within integrated steelworks as feed to the sinter plant or to BOF to utilise useful elements such as Fe, Mn, CaO, MgO [3-7]. Characteristics of compounds in slag and its application are shown in Table 1 [8]. Due to presence of high amount of Ca, it can be used as flux in blast furnace, but presence of high amount of phosphorus in the LD slag makes unsuitable for industrial application as it is a detrimental element for high quality steel products which limits the use of BOF slag in sinter making and Blast Furnace. As steel quality requirements have increased so the need for lower phosphorous levels has led to a reduction in the quantity of steel slag that is recycled.
|
|
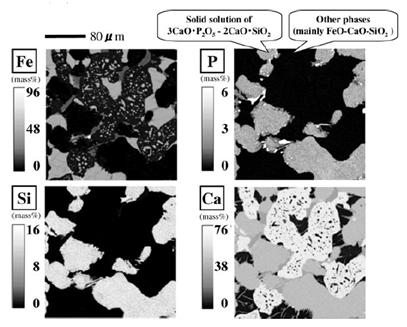 | Figure 1. EPMA mapping image of a BOF slag |
1.1. Magnetic Separation Methods
- Magnetic separation is a process in which magnetically susceptible material is extracted from a mixture using a magnetic force. Slag contains various complex compounds. These compounds have different magnetic property. By taking advantages of these magnetic properties, magnetic and non-magnetic particles may be separated by using a magnetic separator. In this paper above method is used for effective removal of phosphorous containing phases.
2. Experimental
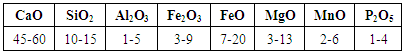
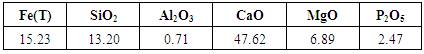
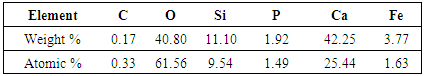
- Basic Oxygen Furnace slag was collected from Steel Authority of India Ltd, Rourkela Steel Plant. This slag sample contains phosphorous oxide as high as 2.47%. The compositional analysis of this sample was done by X-ray fluorescence (XRF) and it is shown in Table 3.
|
|
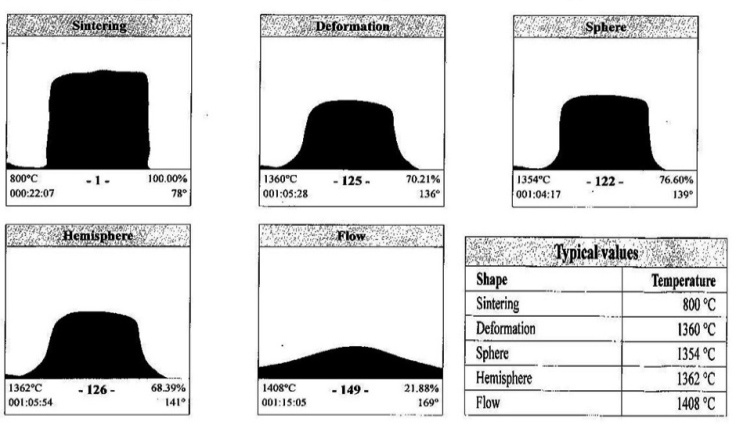 | Figure 2. Shape of slag with variation in temperature |
3. Results and Discussion
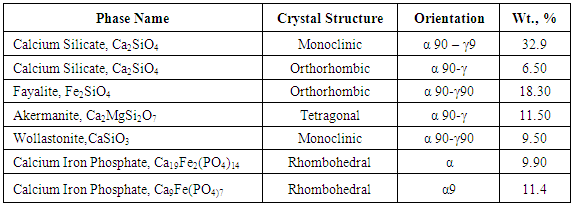
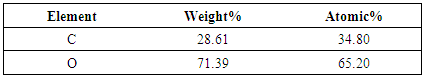
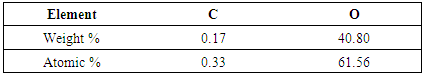
- P2O5 content of slag is generally very high for recycling purpose. Hence this slag needs to be processed. This phos-phorous oxide present in slag was found in various complex phases, Phosphorus containg phase is Calcium Iron Phos-phate (Ca19Fe2(PO4)14 and Ca9Fe(PO4)7). This two forms of phase carries weights around 20% of total wt%. The distri-bution of phosphorous in elemental form was investigated using SEM analysis. SEM image in Figure 3 clearly shows that phosphorus distribution in matrix is random.
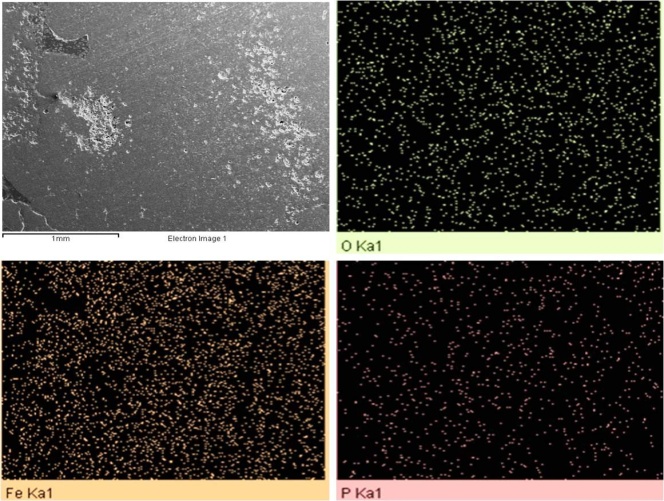 | Figure 3. Distribution of Phosphorous, Iron and Oxygen in Slag |
 | Figure 4. Points in slag where analysis was done |
|
|
|
3.1. Characterization of Treated BOF Slag
- Elemental distribution in treated slag indicates the localized segregation of phosphorus in matrix (Figure 5).
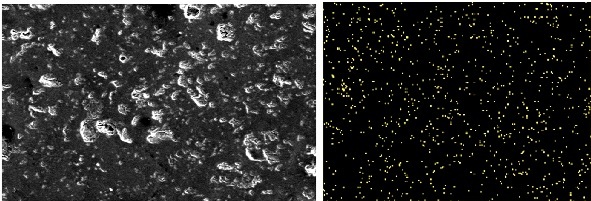 | Figure 5. Elemental distribution of Phosphorous in treated Slag |
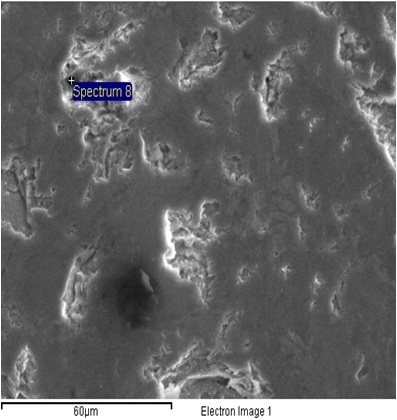 | Figure 6. SEM Point analysis of BOF treated slag |
|
3.2. Magnetic Separation
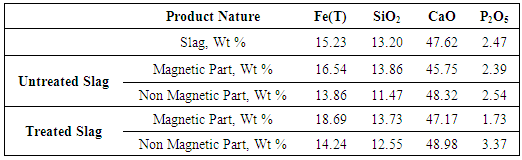
- Magnetic separation was carried out for separating the treated slag using water as a carrier media. After magnetic separation two type of products were obtained viz. a) slag with major magnetic portion b) Slag with fewer magnetic portion. Same experiment was also done without any melting of slag and expected result was found as phosphorus level didn’t get much affected by only magnetic separation. Table 7 shows the phosphorous content in different type of treated slag. Chemical analysis of these products was done for observing phosphorus contents in products and result confirms that it is possible to get phosphorous rich and lean phases using this method.
|
4. Conclusions
- • Dephosphorization of Basic Oxygen Furnace slag is feasible if certain condition is maintained.• This process can benefit steel industries by reducing phosphorus load in input material.• Heating and cooling cycle enabled the segregation of phosphorus in different phases. This segregation is enhanced with slow cooling.• The phosphorous lean phase can be recycled in an iron and steel industry in various ways like feeding material for Blast furnace, sinter plant etc.• Techno-Economical feasibility of this process needs to be checked for industrial scale.• More research is required in this field in order to carry out dephosphorization more effectively.
ACKNOWLEDGEMENTS
- Authors would like to express highest sense of appreciation for guidance of the members of the steel making group and management of RDCIS-SAIL, Ranchi, India. Their efforts support and influence in shaping this work to its completion is greatly valued.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML