-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Metallurgical Engineering
p-ISSN: 2167-700X e-ISSN: 2167-7018
2017; 6(1): 10-17
doi:10.5923/j.ijmee.20170601.02

Effect of Sintering Temperature on the Mechanical Properties for a Cu-Sn-Zn-C Alloy Produced by Powder Metallurgy
Sri Endah Susilowati, Didit Sumardiyanto
Department of Mechanical Engineering, 17 Agustus 1945 University, Jakarta, Indonesia
Correspondence to: Sri Endah Susilowati, Department of Mechanical Engineering, 17 Agustus 1945 University, Jakarta, Indonesia.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This research have been conducted by making of one type of bronze bearing materials Cu-Sn-Zn-C alloy by powder metallurgy method. Powder Metallurgy enables the manufacture of products with controlled levels of porosity in their structure in which the interconnected porosity in the sintered structure is used to hold a reservoir of oil. A self-lubricating sintered bearing is a metallic component with high porosity (20-25% in volume), impregnated in a lubricant oil. The oil contained in the porosity provides a constant lubrication between bearing and shaft, so the system does not need any additional external lubricant. The aim of this research was to determine the effects of sintering temperature on the mechanical properties of a Cu-Sn-Zn-C alloy. The result of this research showed that in 700°C, 750°C and 800°C sintering temperature, the hardness, density, and yield strength values increased, but the rate of wear and porosity values decreased. Meanwhile, at the temperature 825°C, 850°C, 875°C and 900°C, the hardness, density, and yield strength values decreased but the rate of wear and porosity values increased. It happened because at the temperature above 800°C there was rapid diffusion of Sn and Zn to Cu and C alloy which then left a large pore (swelling). Thus, the best value of mechanical properties for a Cu-Sn-Zn-C alloy occurred at the temperature of 800°C with the hardness, rate of wear, density, porosity and compressive strength values were as respectively following 52 BHN, 4.76 x 10-6 mm3/mm, 7.08 gr/cm3, 18,23% and 440 MPa.
Keywords: Cu-Sn-Zn-C Alloy, Metal Matrix Composite, Sinter, Graphite, Mechanical Properties
Cite this paper: Sri Endah Susilowati, Didit Sumardiyanto, Effect of Sintering Temperature on the Mechanical Properties for a Cu-Sn-Zn-C Alloy Produced by Powder Metallurgy, International Journal of Metallurgical Engineering, Vol. 6 No. 1, 2017, pp. 10-17. doi: 10.5923/j.ijmee.20170601.02.
Article Outline
1. Introduction
- Powder metallurgy is the study of the manufacture process of products from metal powder with these following stages: characterization, mixing powders, compacting, until sintering (heating) [1]. Powder metallurgy (P/M) might be chosen as the preferred route for the manufacture of a product. In broad terms, these reasons separate into two categories: (i). Cost effectiveness, P/M is the most cost effective of a number of possible options for making the part and (ii). Uniqueness, some characteristic of the product (e.g. combination of chemical constituents, control over microstructure, control over porosity etc.) can be created by starting from a powder feedstock, which would be very difficult or sometimes impossible in conventional processing [2].Journal bearings are machine elements which provide relative rotational movement between components through sliding movement. Journal bearings must have the expected features, such as low coefficient of friction, high wear resistance, high load capacity and corrosion resistance, good thermal conductivity, low thermal expansion and embedding foreign particles, easy workability and low cost [3]. To obtain high mechanical and tribological properties, journal bearings are produced by alloying in powder metallurgy method [4-6]. To produce porous metallic products, powder metallurgy is the most common method by which the level of porosity and the size distribution of pores are controlled. These properties of the produced parts depends on material composition, pore size and porosity distribution. Pore type, amount, distribution and shape of powder are important to improve the conditions for self-lubricating bearings and regulate the amount of oil impregnated [7]. Porous sliding bearings, due to their oil deposition advantage of about 25% of their volume in the pores, are produced by sintering of metal powders compressed under a certain pressure [8, 9]. During service, oil in pores comes to the surface for lubrication. This is one of the most desired features in bearings. Therefore, P/M techniques are utilized to impregnate oil into machine parts having no possibility of continuous lubricating. Journal bearing materials which are made from copper-based materials have been widely due to properties such as good corrosion resistance, high thermal and electrical conductivity, self-lubrication and good abrasion resistance [10-12].Graphite is always added to improve lubrication. Bronze bearing with the addition of graphite suitable for conditions with heavy loading, high load and high temperature. Graphite added in the process of making bearings serves as lubrication [1]. For bronze bearing, the material composition has 90% Cu-10% Sn with the addition of graphite to 1.5% [2]. Addition of graphite is used to modify friction during application [13]. Additionally, the addition of graphite to copper-tin alloys is also used to control dimensional changes [14].The type of bearing to be made in this study was a type of bronze bearing using weight fraction of 88% Cu, 9% Sn, 3% Zn, and 1.5% C graphite with the different sintering temperature 700°C, 750°C, 800°C, 825°C, 850°C, 875°C and 900°C. The bronze bearing materials has pores, which serves as a storage place lubricant (oil reservoir) so that once inserted into the bearing lubricant, the bearing lubricant needs not be given back because the lubricant is already in the pores of the bearing.
2. Experimental
- Copper, tin, zinc powders with a particle size of 65 μm, 65 μm and 42 μm, respectively were used as bronze bearing or as a matrix and graphite powder with 50 μm size was mixed in the blender for 5 minutes. The composition of Cu: Sn: Zn is 88: 9: 3 and the addition of graphite with 1,5% weight fraction. All the powders were prepared then compacted with a pressure of 200 bars for 30 seconds. Green compact with 10 grams of weight each was then sintered in Carbolite furnaces with sintering temperatures varied from 700°C, 750°C, 800°C, 825°C, 850°C, 875°C and 900°C for 5 minutes then furnace cooled. To prevent thermal shock, heating rates were set at 100°C per minute, and held for 10 minutes at 500°C. All samples were characterized both mechanical properties and microstructural analysis.
3. Result and Discussions
3.1. Hardness Test
- The hardness testing results that the hardness values increased with the increasing sintering temperature and achieved the optimum hardness at temperature 800°C, which amounted to 52 BHN, then the value of hardness decreased significantly with the increased sintering temperature are shown in Figure 1.
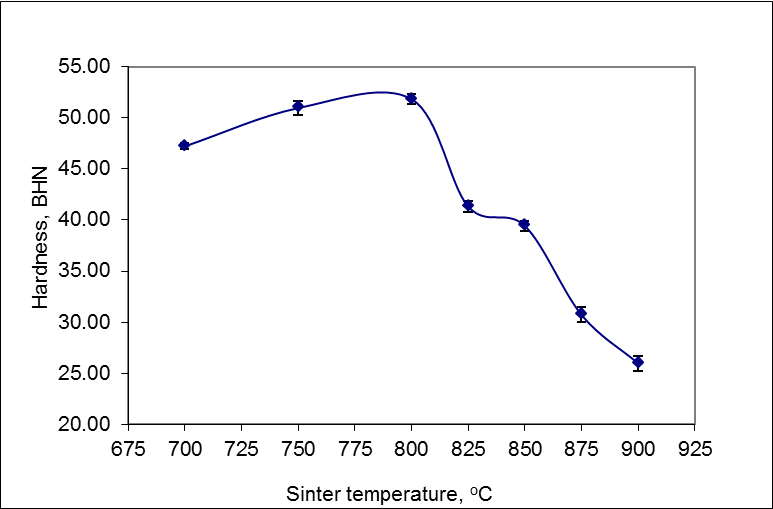 | Figure 1. Effect of sintering temperature on the hardness |
3.2. Wear Test
- The test result that the rate of ware decreased until the minimum limit. That was 4,76 x 10-6 mm3/mm in temperature 800°C, then increase from 4,78 x 10-6 mm3/mm in temperature 825°C until 6,70 x 10-6 mm3/mm in temperature 900°C (Figure 2).
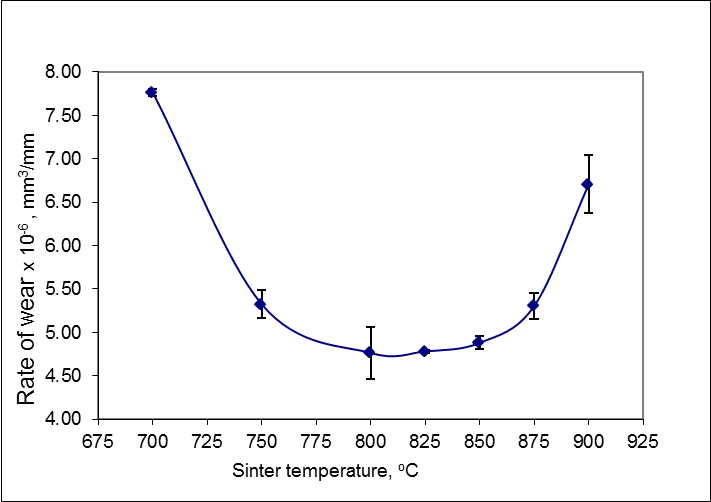 | Figure 2. Effect of sintering temperature on the rate of wear |
3.3. Density Test
- Based on density test, the value of density had increase untill at the temperature of 800°C at 7.08 gr/cm3, and the density decreased from the temperature of 825°C at 6.80 gr/cm3 to the temperature of 900°C amounted to 6.27 gr/cm3 (Figure 3).
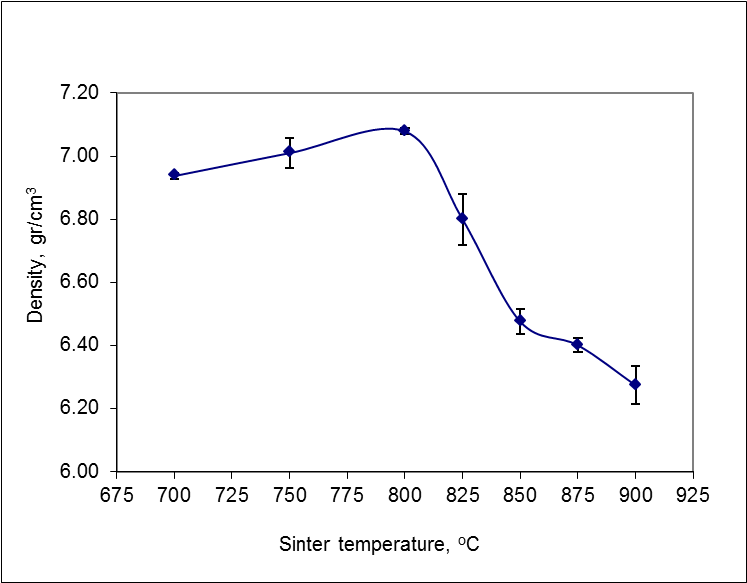 | Figure 3. Effect of sintering temperature on density |
3.4. Porosity Test
- Porosity value was inversely proportional to the density. The increase of density was followed by the decrease in porosity. Figure 4 shown the relationship of porosity and the sintering temperature indicating the decrease in porosity values from 700°C up to 800°C. This happened because the pores between grains of Cu or graphite were filled by liquid phase Sn and Zn so that the pore was reduced. Before sintering, many pores closed by the point of contact between the particles and pore surface gathered around grains. Then, as a result of the sintering process with sintering temperature 800°C, there was rearrangement of Sn and Zn liquid phase filling the pores between Cu or graphite so that the pore was reduced.
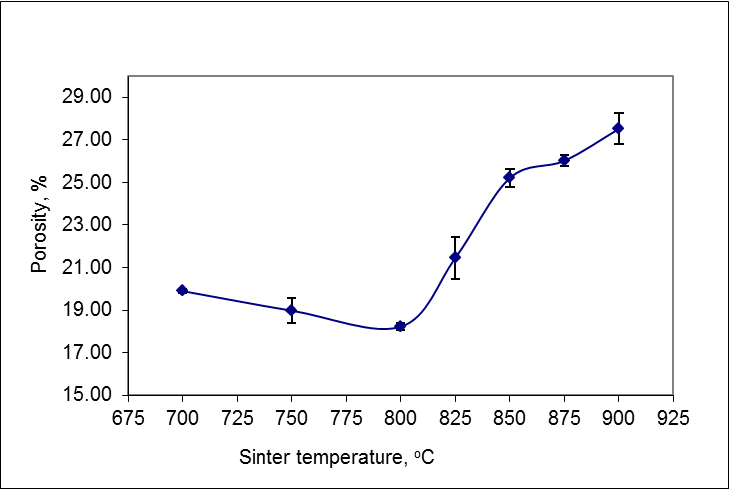 | Figure 4. Effect of sintering temperature on porosity |
3.5. Compressive Strength Test
- The compressive strength increased up to a temperature of 800°C with a value of 440 MPa, then decreased from 825°C temperature of 419 MPa at the temperature up to 900°C amounted to 275 MPa (Figure 5).
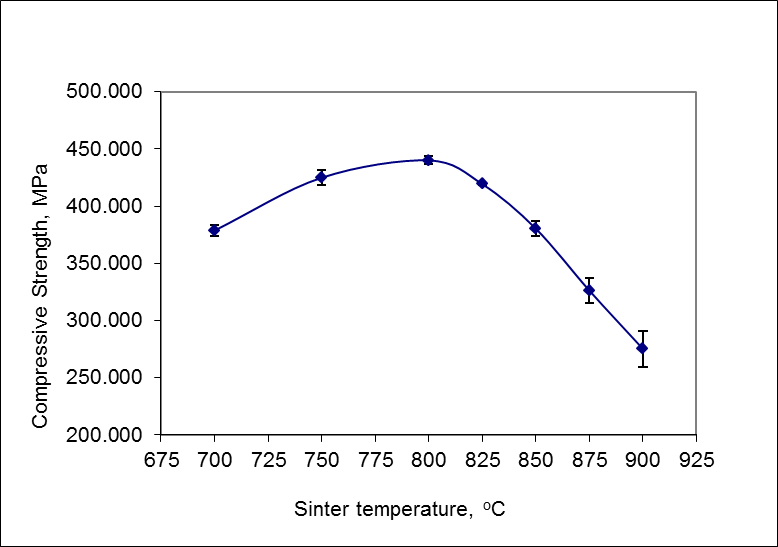 | Figure 5. Effect of sintering temperature on compressive strength |
3.6. Microstructure Observation
- The result of microstructural photograph can be seen that with increasing of sinter temperature from 700°C to 800°C happened pore reduction (Figure 6a,b) then with sinter temperature rise 850°C to 900°C happened pore enlargement (Figure 6c,d). This might indicate a densification followed with downsizing as the pore was filled by pore liquid phase up to the temperature 800°C. Meanwhile, at the temperature of 850°C up to 900°C (Figure 6c,d) the size of the pores got larger due to their swelling.
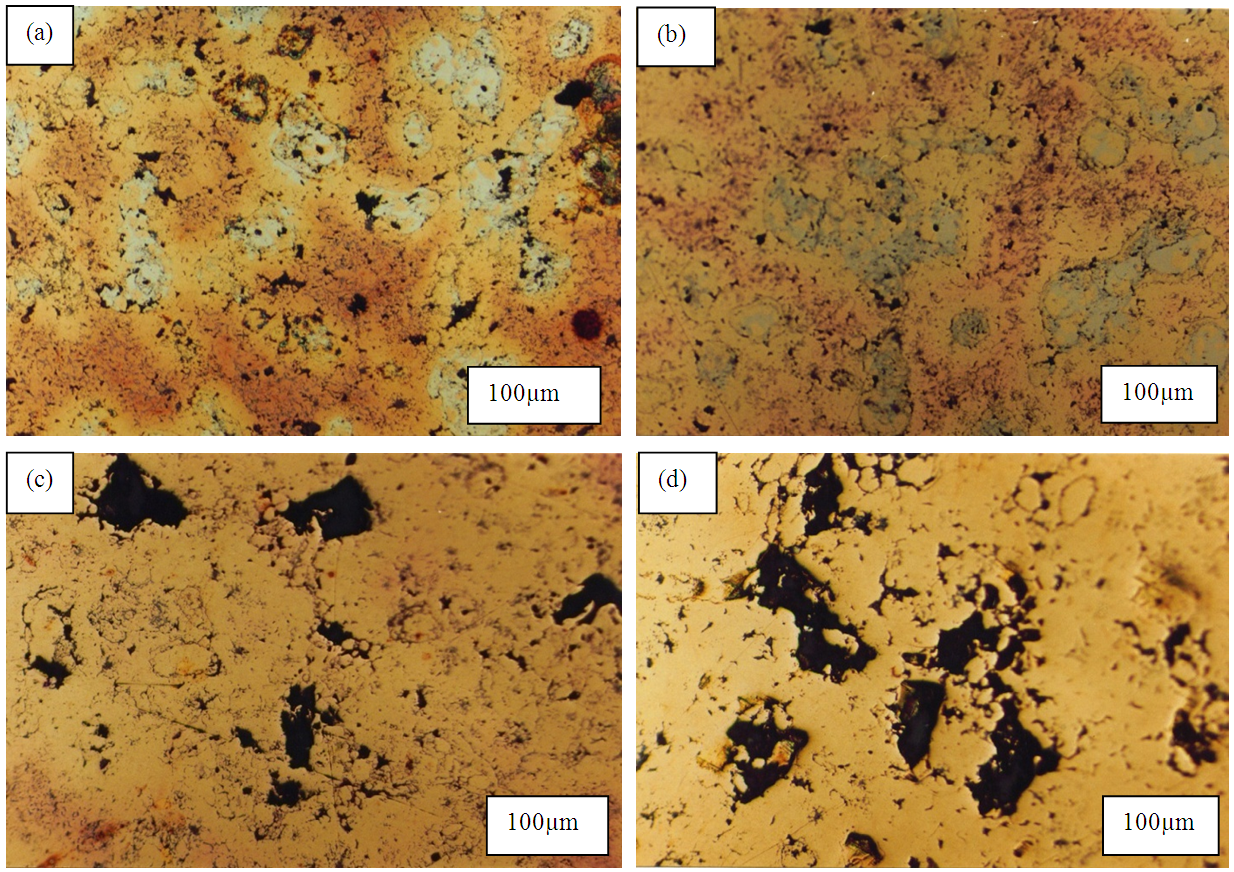 | Figure 6. Microstructure sample with sintering temperature at (a) 750°C (b) 800°C (c) 850°C (d) 900°C |
3.7. SEM Observation
- Observation with SEM showed non sinter graphite sample packs and evenly spread with a lot of void around the graphite (Figure 7). In the observation by using an optical microscope, it was not apparent yet.
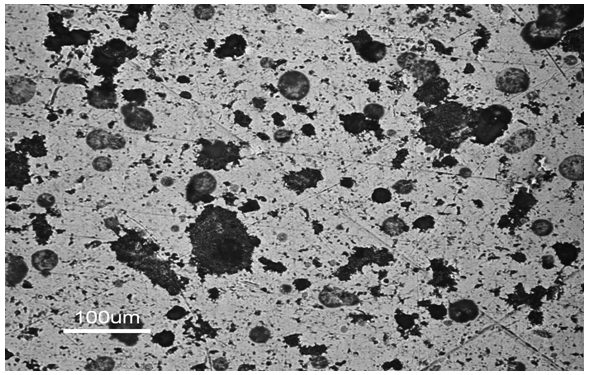 | Figure 7. Non sinter microstructure sample with SEM |
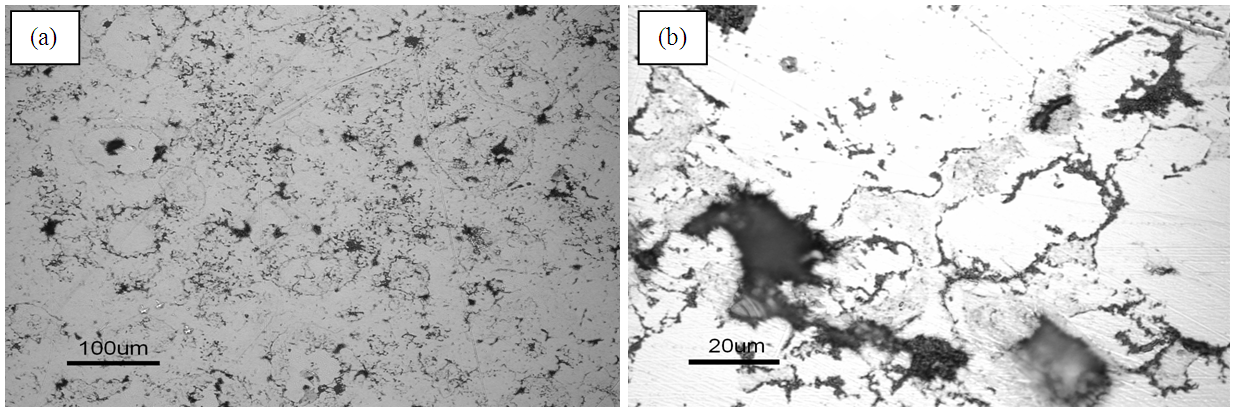 | Figure 8. Microstructure sample sintering with SEM at (a) 800°C (b) 850°C |
3.8. EPMA (Electron Probe Micro analyzer) Observation
- Microstructure results in compaction which still formed the bulk of green, not yet sintered, as observed by EPMA showed that they gathered Sn concentration (Figure 9a). This was due to the condition of non sinter Sn and Zn which served as a wetting agent had not experienced the liquid phase. The strength properties of the material non-sinter powder metallurgy products were more affected by their mechanical strengthening (cold weld and mechanical interlocking) [21].The results of EPMA with sintering temperature 800°C showed Sn melted and diffused into the Cu forming CuSn phase (Figure 9b). At the sintering temperature above 800°C up to 900°C the size of the pores got larger and the number of pores significantly increased. This pore enlargement signified rapid diffusion of Sn to Cu and resulted in decreased material strength [16].
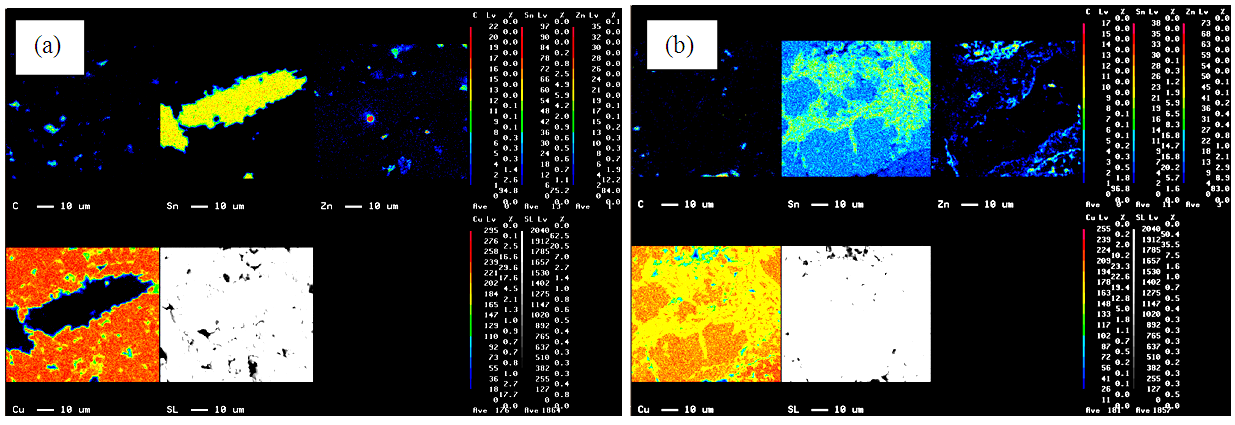 | Figure 9. Microstructure sample EPMA at (a) Non Sinter (b) 800°C |
3.9. Characterization by XRD
- The diffraction pattern sample Cu-Sn-Zn-C alloy with non sinter treatment resulted in two phases, namely Cu and Sn (Figure 10a). For Zn phase that was not identified, this occurred because the concentration of Zn was small (only 3%). In the non-sintered samples, the alloy has yet to form so that there were only pure phases as in their constituent particles. Cu phase was indicated by the diffraction peaks with orientation hkl (111), (200), (220), (311) and (222), the space group F - centered cubic (Fm-3m) and the lattice parameters a = b = c = 3.615 °A. For Sn, it was indicated by the orientation hkl diffraction peak (200) and (411), the space group I - centered tetragonal (141 / amd) with lattice parameters a = b = 5,819 °A and c = 3.175 °A.Diffraction Patterns and Crystal Structure for Sinter Sample 700°CSample identification with sintering temperature of 700°C, identified the dominant Cu phase and just a few of Cu3Sn phase (Figure 10b). The visible Cu phase is still the same as the phase in the non-sinter sample and is shown by the diffraction peaks with the hkl orientations (111), (200), (220), (311) and (222) with the F-centered cubic space group (Fm-3m ) and lattice parameters a = b = c = 3.615°A. For Cu3Sn phase represented by diffraction peak with hkl orientation (0160), (002) and (2120)) with group space Cmcm and lattice parameter a = 2.749°A, b = 2.749°A and c = 4.322°A.At sintering temperature 700°C there is still Cu phase and there is a few of Cu3Sn. This is because at that temperature there is only a small amount of Sn and Zn diffusing into Cu, especially with a short sinter resistance time (5 minutes) has not formed alloy.Diffraction Patterns and Crystal Structure for Sinter Sample 800°CThe process of sintering the Cu-Sn-Zn and graphite with a sintering temperature of 800°C produced Cu20Sn6 dominant phase, besides there was still Cu phase (Figure 10c). For Cu20Sn6 phase, it was indicated by the orientation of the field hkl peak (300), (113) and (106) with the space group P63 and lattice parameters a = b = 7.330 °A and c = 7.864 °A. While the Cu phase was indicated by the orientation hkl diffraction peak (111), (200) centric space group F - centered cubic (Fm-3m) and the lattice parameters a = b = c = 3.615 °A. At sintering temperature of 800°C a liquid phase sintering mechanism takes place through the diffusion of atoms where the liquid phase Sn and Zn fill the pores between Cu and graphite powder and also moisten the Cu grain boundary to form a bond [13, 16]. Sn diffuses with Cu to form Cu20Sn6 alloys, in the presence of these alloys also causing increased hardness of material properties.Diffraction patterns and Crystal Structure for Sinter Sample 850°CThe diffraction pattern of samples Cu-Sn-Zn and graphite with sinter treatment of 850°C can be seen in Figure 10d. Samples with sintering temperature of 850°C, they produced dominant Cu39Sn11 phase and there was still Cu phase. For Cu39Sn11 the phase was indicated by the orientation of the field hkl peak (660), (844) and (888) centric space group F - centered cubic (F43m) and the lattice parameters a = b = c = 18.01 °A. While the Cu phase is indicated by the orientation hkl diffraction peak (111), (200) and (220) centric space group F - centered cubic (Fm-3m) and the lattice parameters a = b = c = 3,607 °A. At 850°C there is a phase change from Cu20Sn6 to Cu30Sn11. At this temperature Zn starts to evaporate (boiling point Zn = 906°C) to form Zn oxide on the sample surface.
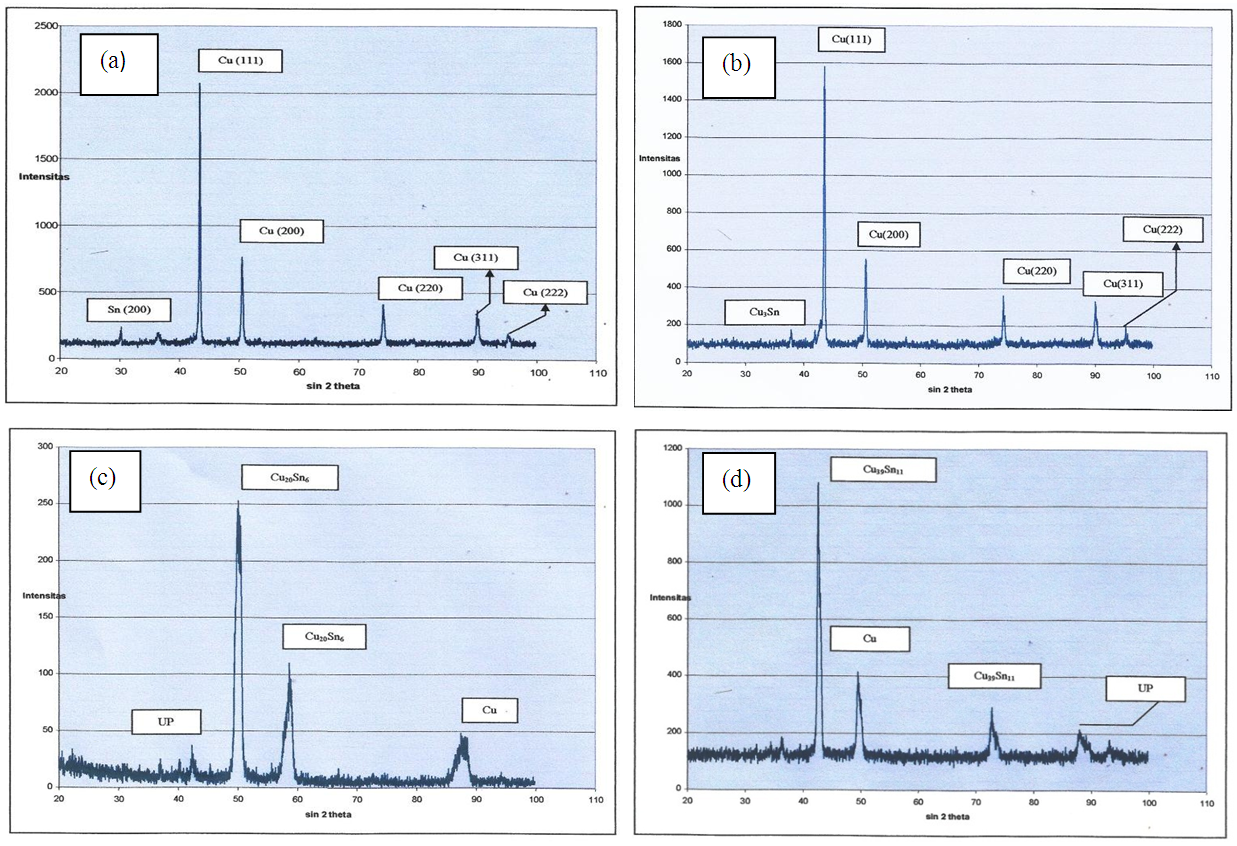 | Figure 10. Diffraction patterns and crystal structure at (a).non sinter (b) 700°C (c) 800°C (d) 850°C |
4. Conclusions
- This study concluded that:1. One type of bronze bearing material Cu-Sn-Zn-C Alloy can be made by powder metallurgy method.2. Sinter temperatures affect on the mechanical properties of Cu-Sn-Zn-C Alloy material.3. At sinter temperature 800°C, the mechanical properties of Cu-Sn-Zn-C Alloy reach optimum value. At this temperature, the values of hardness, density, compressive strength, porosity and wear rate are respectively: 52 BHN, 7.08 gram / cm3, 440 MPa, 4.76 x 10 -6 mm3 / mm and 18.23%.4. However, the increase in the sintering temperature above 800°C (in this research 825°C, 850°C, 875°C and 900°C) cause the hardness value, density and compressive strength decrease but the rate of wear and porosity value increase.5. From the results of microstructure photo observations, visible pore size is getting smaller with the increasing temperature up to 800°C. After 800°C, the size of pores gets larger. This is due to densification occurs and the diffusion between Sn and Zn (liquid phase) in Cu and graphite. The function of Sn and Zn is as a wetting. From the results of XRD, it shows that Sn diffuses into Cu, it is shown by the appearance of Cu20Sn6 phase at the temperature of 800°C and Cu39Sn11 phase at the sintering temperature above 800°C. The Cu20Sn6 phase is suspected of causing the material strength to increase.6. At the sintering temperature above 800°C, Sn diffuses quickly and leaves the pores causing swelling (enlarged pore size) and the material strength to decrease.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML