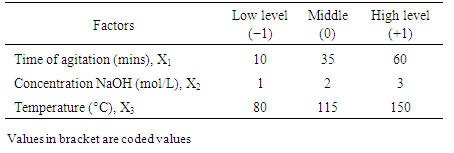-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Metallurgical Engineering
p-ISSN: 2167-700X e-ISSN: 2167-7018
2017; 6(1): 1-9
doi:10.5923/j.ijmee.20170601.01

Decreasing Yield and Alumina Content of Red Mud by Optimization of the Bauxite Processing Process
Cornelius Tsamo1, 2, Guillaume Patrice Kofa3, Richard Kamga2
1Department of Chemistry, Higher Teachers’ Training College, University of Maroua, Cameroon
2Laboratoire des Matériaux et Chimie Inorganique Industrielle, University of Ngaoundéré, Cameroon
3Department of Hydraulics and Water Management, Higher Institute of the Sahel, University of Maroua, Cameroon
Correspondence to: Cornelius Tsamo, Department of Chemistry, Higher Teachers’ Training College, University of Maroua, Cameroon.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Bauxite produces 60-120 million tons of toxic red mud annually during its processing to alumina. Thus, in this work, the modeling of the bauxite processing process to reduce quantity of red mud produced and with low alumina content was envisaged. Bauxite from Minim-Martap was processed by adapting the Bayer process under laboratory conditions. This process was then optimized to obtain conditions that yield less red mud with reduced alumina content. This was done using the Doehlert experimental design with three factors: stirring time, sodium hydroxide concentration and temperature. X-ray diffraction (XRD) and X-ray fluorescence (XRF) were used to characterize obtained alumina, red mud and crushed bauxite. Model process equation obtained from optimization results shows that increasing the tested parameters lead to production of low amount of red mud (temperature and sodium hydroxide concentration were the main factors that influenced the process). The model obtained described the process adequately with a 92% coefficient of determination, low absolute average deviation of 0.004 and strong agreement between theoretical and experimental responses. The optimum conditions gave a red mud yield of 86% with 13% alumina compared to a non-optimized process with 98% red mud yield and 16% alumina content. The fact that increasing tested parameters reduced red mud yield however, implies high amount of alumina is produced, thus a significant economic and environmental advantage for the aluminum industry. It was concluded that optimization of bauxite processing process reduced red mud produced and its alumina content.
Keywords: Bauxite, Bayer process, Experimental design,Minim-Martap, Red mud
Cite this paper: Cornelius Tsamo, Guillaume Patrice Kofa, Richard Kamga, Decreasing Yield and Alumina Content of Red Mud by Optimization of the Bauxite Processing Process, International Journal of Metallurgical Engineering, Vol. 6 No. 1, 2017, pp. 1-9. doi: 10.5923/j.ijmee.20170601.01.
Article Outline
1. Introduction
- Red mud is the caustic by-product stream from alumina production via the Bayer process, often colloquially referred to as red mud or bauxite residue or Bayer process tailings [1, 2]. About 90% of raw bauxite ore goes into the waste as alkaline red mud slurry during processing [3] as for every ton of alumina produced, between 1-2 tons (dry weight) are produced depending on the bauxite source and alumina extraction efficiency [4, 5]. Globally, about 60-120 million tons are produced annually [6]. This may lead to serious pollution of the surrounding soil, air and groundwater due to its high pH (10-13) [7, 8]. Depending upon jurisdiction, untreated bauxite residue may be classified as hazardous primarily due to its alkalinity rather than heavy metal or naturally occurring radionuclide content [9]. However, red mud contains a number of valuable metals and minerals (from parent bauxite and those introduced during the Bayer process) like aluminium, iron, silica, calcium, titanium and some minor constituents namely: Na, K, Cr, V, Ni, Ba, Cu, Mn, Pb, Zn etc. The typical constituents of red mud (% w/w) are: Fe2O3 (30-60%), Al2O3 (10-20%), SiO2 (3-5%), Na2O (2-10%), CaO (2-8%), TiO2 (trace-10%) [10, 11], depending on the type and quality of ore used and the process parameters. Red mud has been used for metal recovery, building material, ceramics production, catalysis, soil amendment, pigments and paints, water treatment [8-9,12-16] etc.Bauxite the primary source of over 99% of world aluminium [17] is a naturally occurring mixture of minerals rich in hydrated aluminum oxides (40-60%). The major impurities of bauxite are the oxides of Fe, Si, and Ti and trace amounts of metals which constitute red mud [11]. The most important Al- containing bauxite minerals are gibbsite [Al(OH)3], boehmite [γ-AlO(OH)], and diaspore [α-AlO(OH)]. Based on their mineralogy, bauxites can be divided into two types; Lateritic bauxites, are predominately gibbsite and to a lesser extent boehmite and comprise approximately 90% of the world's exploitable bauxite reserves while karst bauxites are principally boehmite, and diaspora [18]. Though alumina can be produced from bauxite under alkaline conditions using lime (Lime Sinter process), sodium carbonate (Deville Pechiney process), at high temperature in reducing environment with presence of coke and nitrogen (Serpeck process), the alkalinisation by the use of sodium hydroxide (Bayer process) is the most economical process which is employed for purification of bauxite if it contains considerable amount of Fe2O3 [19]. It is responsible for 90% of the world's alumina production from bauxite [20]. It is a high temperature and high pressure selective dissolution process extracting gibsitte and/or boehmite from bauxite by dissolving these constituent in hot concentrated NaOH and 106-240°C and at 1-6 atm pressure [9, 20-22]. After bauxite dissolution or digestion, the NaAl(OH)4- rich solution is separated from the remaining less soluble materials such as iron oxide and silica, known in the industry as “red mud or bauxite residue” [20]. NaAl(OH)4- is precipitated to give Al (OH)3 which is calcined at 1,000–1,200°C to give Al2O3. Lateritic bauxites are easier to digest than karst bauxites using less severe conditions of caustic concentration, temperature and/or holding times [18].Bayer process is entirely a large scale industrial process, unfortunately the nature and scope of the information about it is owner and/or refinery specific and not consistent in either form or content. As, each refinery has unique operating details with respect to red mud technologies, management and engineering practices [23] thus, placing a severe limitation on the ability to collect systematize and interrogate information on the process. The above review shows that the process is influenced principally by the parameters; holding time, bauxite type, sodium hydroxide concentration and temperature and pressure. The improvement of process conditions can reduce the about 90% of raw bauxite ore that goes into the waste and yields more alumina. Published information on the laboratory processing of bauxite in general and particularly bauxite from Cameroon as well as the optimization of the process is very scarce. It is estimated that Cameroon has the 6th world bauxite reserves [24], with approximately 1.8 billion tons from which 1 billion tons are estimated for the two groups of deposits situated in the Minim-Martap and Ngaoundal [25]. But there is no bauxite exploitation activity in Cameroon yet. This work is thus, aimed at adapting the Bayer process at laboratory level to process bauxite from Minim-Martap and optimizing the process parameters in view of identifying process conditions that yield less amount of red mud with improved properties (reduced alumina content) and help raise awareness on risk of bauxite processing in anticipation of lateritic bauxite [26] processing to start in Cameroon.
2. Materials and Methods
2.1. Sampling of Bauxite and Its Preparation for Processing
- Samples, mainly red bauxite were collected at Sabal Haleo (06°27’27’’ N 12° 59’ 28’’ E) figure1a from bore holes (about 10 meters deep) figure 1b, dug by Cameroon Alumina Limited (CAL) during exploration.
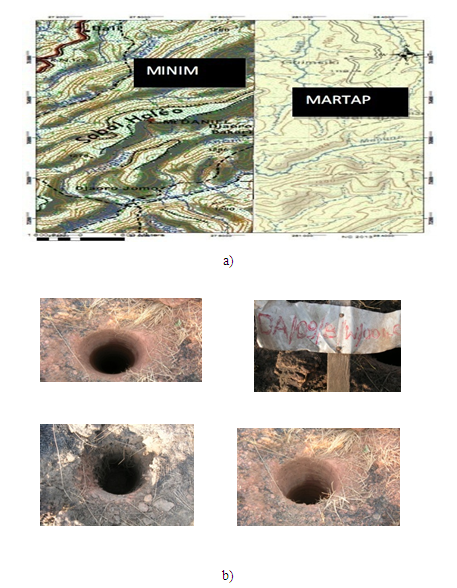 | Figure 1. a) Map of the sampling zone b) Bore holes where bauxite was collected |
2.2. Processing of Minim-Martap Bauxite
- A trial extraction process was performed using the procedure described by Benhamou et al., 2008 and Excoffier, 2009 [27, 28]. Results of the trial process (which gave 98% red mud yield, rich in alumina) oriented the optimization of the extraction. For trial process, 20 g of finely crushed and grinned bauxite were mixed with a prepared 120 mL solution of 3M NaOH in a 250 mL capped Erlenmeyer flask. This mixture was stirred (300tr/min) for 10 minutes and then, heated at 80°C for 20 minutes (on a Thermo Scientific Cimarec stirring hot plate, model: SP 131320-33). After this period, the slurry was allowed to cool down to laboratory temperature and the slurry was filtered over Whatmann filter paper N° 1. Red mud was collected on the filter paper while the filtrate was collected in a 500 mL Becker where a prepared 3M solution of HCl was added drop wise to the filtrate to a pH between 5 and 6 (which is the zone of stability for aluminium hydroxide), measured using a Hach HQ 40D multi pH meter. The precipitate obtained was filtered and dried overnight at 120°C and calcined at 1000°C (in a muffle oven for 3 hours) to obtain alumina. Red mud obtained was washed with distilled water several times and dried at 100°C overnight, stored at ambient temperatures and used without further treatment.
2.2.1. Optimization of the Minim-Martap Bauxite Processing Process
- The Doehlert design was used because of its numerous advantages over other designs [29]. Particularly, it permits to follow a sequential manner in studying a response surface of second degree. Also the matrix is flexible for adding new parameters or extending experimental domains, without restarting the experiments that has already been done. Moreover, polynomial equations with and without interaction are used as models. Then, the coefficient of determination (R2) and absolute average deviation (AAD) are used to investigate the adequacy of the proposed models. The selection of the experimental design was based on the assumption that bauxite processing process is affected by 3 variables: stirring time (X1: 10 - 60 min), sodium hydroxide concentration (X2: 1 -3 mol/L), digestion temperarure (X3: 80 - 150°C), Table 1.
|
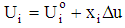 | (1) |
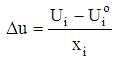 | (2) |
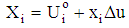 | (3) |
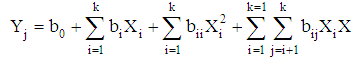 | (4) |
2.3. Characterization of Bauxite, Alumina and Red Mud
- The alumina obtained from trial process was analyzed by X-ray diffraction (XRD) to determine the efficiency of the extraction process. Chemical composition of red mud obtained under the two processing conditions (trial and optimum) was determined by X-ray Fluorescence (XRF) with aim of controlling red mud alumina content from the two conditions. The chemical composition of the bauxite used was also determined by XRF. The mineral composition of red mud was also characterized by XRD.
3. Results and Discussion
3.1. Modeling of the Process
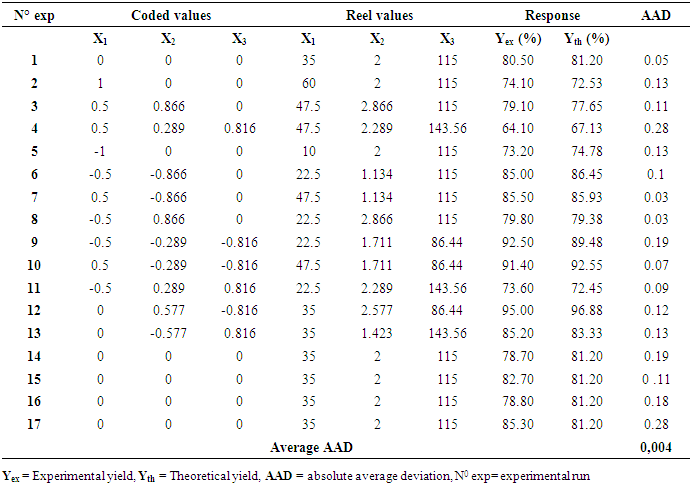
- The design matrix in coded and real values, together with the experimental values of the responses (red mud yield in %), theoretical yields of red mud generated from statgraphics and calculated values of ADD obtained from equation 5 below at different conditions, are presented in table 2.
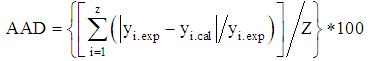 | (5) |
|
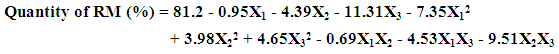 | (6) |
3.1.1. Validation of the Model
- The value of coefficient of determination for the red mud yield from Minim-Martap bauxite processing is 92.95%. The value of R2 shows that the proposed model is adequate. Mendenhall, 1975 [31] reported that the closer the value of R2 to the unity, the better the empirical models and the actual data. In fact, Joglekar and May, 1987 [32] suggested that, for a good fit of a model, R2 should be at least 80.0%. On the other hand, the low absolute average deviation (AAD) value red mud yield from Minim-Martap bauxite processing (0,004) confirms the adequacy of the model as it must be as small as possible [33]. The validity of the model equations was also tested by drawing a regression line between experimental and theoretical responses for red mud yield (figure 2). The agreement (or low residues) between the responses also validates this model.
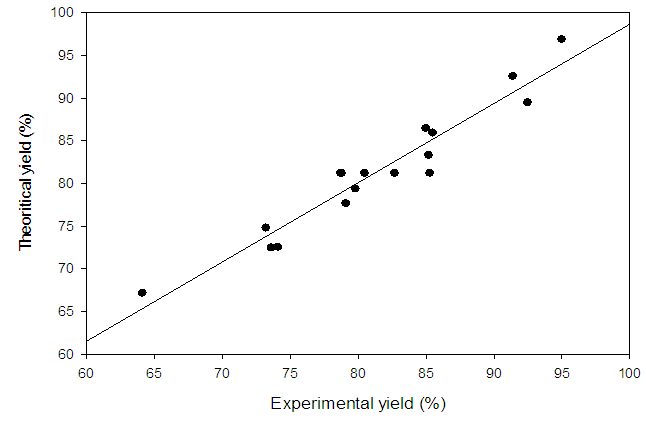 | Figure 2. Correlation between experimental and theoretical yields (%) of red mud from Minim-Martap bauxite |
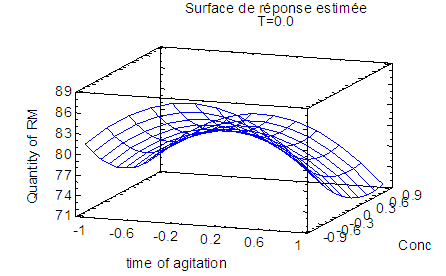 | Figure 3. Response surface graph showing the variation of red mud yield from Minim-Martap bauxite processing as a function of stirring time and sodium hydroxide concentration |
3.2. Characterization
3.2.1. Mineral Composition by XRD
- The XRD diffraction patterns of Al2O3 from the trial process is shown in figure 4 below, showing the presence of high amounts of alumino-silicate minerals (Kaolinite, Al2Si2O5(OH)4 (18.51%), Quartz, SiO2 (19.27%), Anorthite, CaAl2Si2O8 (40.10%), and only 12.90% of Al2O3.
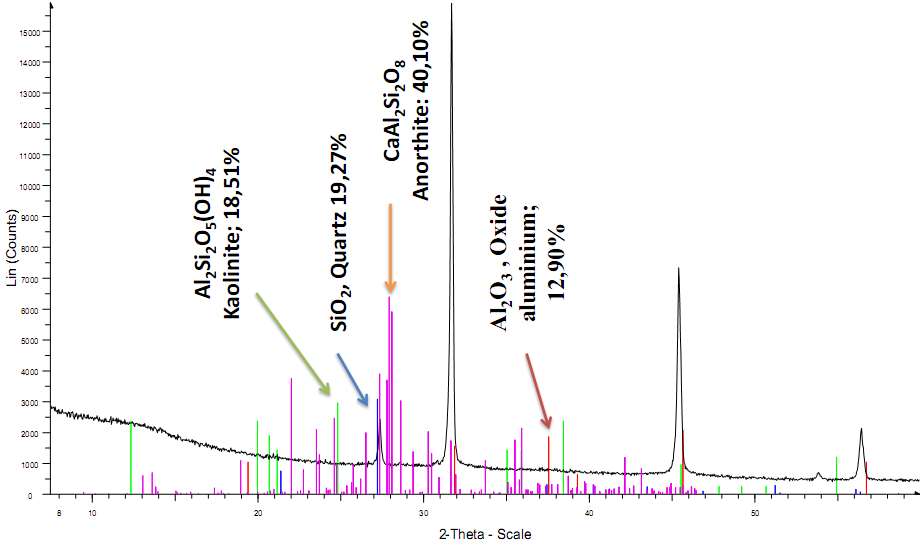 | Figure 4. XRD pattern of alumina obtained from Minim-Martap bauxite |
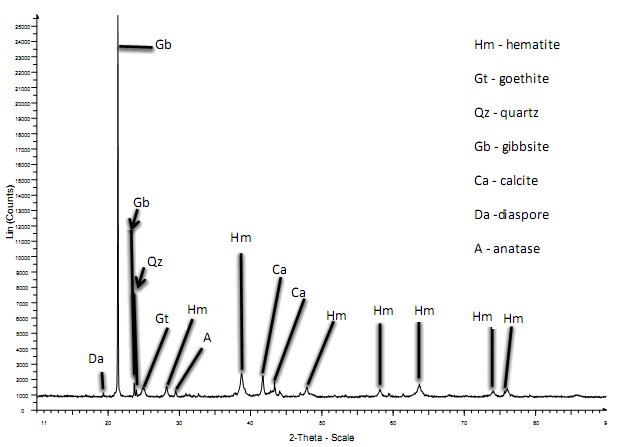 | Figure 5. XRD pattern of red mud obtained from Minim-Martap bauxite |
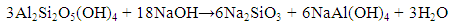 | (7) |
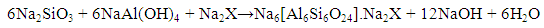 | (8) |
3.2.2. Chemical Composition (Expressed as Oxide) by XRF
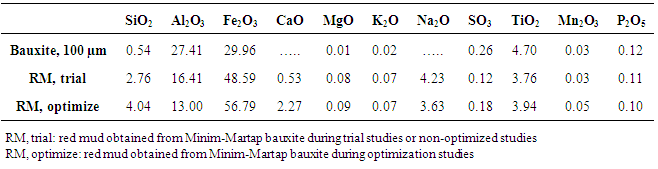
- The chemical composition of bauxite (100 µm), red mud produced by trial process and under optimum conditions (using 100 µm bauxite particles) is shown in Table 3 below. While the quantities of trace elements remain almost the same after bauxite processing, those of Si, Al, Fe, Na and Ca show significant variations from those of the parent bauxite. It is also seen from this table that red mud obtain under optimum conditions has smaller amount of Al2O3 (13%) compared to 16% of under trial conditions and high amounts of Fe and Si oxides (56 and 4% respectively) compared to 48 and 2.76% respectively for trial process. Thus, optimization improves Bayer process by reducing alumina content in red mud which is the main goal of the process. Na2O and Cao are only introduced in red mud during bauxite processing as it is absent in bauxite. It probably represents the DSP formed during predesilication.
|
4. Conclusions
- The optimization of the processing of bauxite from Minim-Martap under laboratory conditions to obtain low quantity of red mud with reduced alumina content was investigated. From the results obtained, the following conclusions are drawn: - Increasing the stirring time, temperature and sodium hydroxide concentration leads to production of low amount of red mud.- The amount of red mud decreased from 98% in a non-optimized process to 86% with optimization.- Red mud alumina content also decreased from 16% in a non-optimized process to 13% with optimization.- Temperature and sodium hydroxide concentration are the main factors that influenced the process. - The model obtained described the process adequately as evident from values of coefficient of determination (92%), low absolute average deviation (0.004) and strong agreement between experimental and theoretical responses.Results of this study constitute the long-term objective on red mud management from production to disposal because each refinery has unique operating details with respect to red mud technologies, management and engineering practices thus, placing a severe limitation on the ability to collect systematize and interrogate information on the process.
ACKNOWLEDGEMENTS
- The authors express their special gratitude to the European membrane institute, France, Metal-Catalysis of the University of Johannesburg, South Africa and Cemencam Cameroon where XRD and XRF analyses were conducted. We also thank the inhabitants of Minim and Martap who guided us through the collection of the bauxite.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML