-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Metallurgical Engineering
p-ISSN: 2167-700X e-ISSN: 2167-7018
2016; 5(2): 31-36
doi:10.5923/j.ijmee.20160502.03

Effect of Boron-to-nitrogen Ratio and Quenching Media on the Properties of Low Carbon Low Manganese Unalloyed Steel
Pratiksha Pandey , Anjana Deva , B. K. Jha , Santosh Kumar , D. Karmakar , A. K. Bhakat
RDCIS, SAIL, Ranchi, India
Correspondence to: A. K. Bhakat , RDCIS, SAIL, Ranchi, India.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The present work was carried out to understand effect of boron to nitrogen ratio and the different quenching media in low carbon low manganese unalloyed steel. Tensile samples of hot rolled sheets were heat treated in laboratory muffle furnace to 890°C and quenched in oil and water. Mechanical properties and microstructure of quenched samples were analysed. A decreasing trend of YS/UTS ratio observed after quenching irrespective of the quenching media. It has been observed that boron and cooling rate in combination improves the hardenability and increase the UTS value. Boron played an important role in contributing microstructure evolution with different quenching media and thereby resulted excellent combination of properties in cost effective low carbon unalloyed steel. Good combination of properties observed in water quenched as well as oil quenched steel samples with low B/N ratio. Quenched samples were further normalized at 920°C and properties were compared with as rolled boron free steel and results shows improvement in properties in terms of lower YS/UTS value with boron addition. The reason behind may be formation of contributing phases which are retained in the microstructure even after normalizing. Drop in strength values when compared with properties of low carbon boron added hot rolled steel revealed more drop in case of water quenching than that of oil quenching.
Keywords: Low carbon boron added steel, Quenching, Normalizing, Drawability
Cite this paper: Pratiksha Pandey , Anjana Deva , B. K. Jha , Santosh Kumar , D. Karmakar , A. K. Bhakat , Effect of Boron-to-nitrogen Ratio and Quenching Media on the Properties of Low Carbon Low Manganese Unalloyed Steel, International Journal of Metallurgical Engineering, Vol. 5 No. 2, 2016, pp. 31-36. doi: 10.5923/j.ijmee.20160502.03.
Article Outline
1. Introduction
- Significant progress has been made towards the development of new steel products with improved attributes by addition of micro-alloying elements in the past [1-3]. The properties attained in the final product depend on presence of small additions of niobium (Nb), vanadium (V), titanium (Ti) or boron (B), which by precipitating in the form of carbide and carbo-nitride, can give precipitation hardening and / or inhibition of grain growth. Out of these elements boron is a unique one, which can increase or decrease the hardness of steel depending on presence of titanium or zirconium (Zr), nitrogen (N) content and process conditions i.e. austenitising temperature, cooling rate etc. [4-11]. Carbon and low/high alloyed steels are quenched in water, oil or other medium to increase its hardenability. This process comprises soaking of a material at a high temperature, above the recrystallization phase, followed by a rapid cooling process to obtain certain desirable material properties associated with a crystalline structure or phase distribution. Although boron as a cost effective hardenability agent for low alloyed quenched and tempered steel has long been recognized [12, 13], amount of boron, carbon and other alloying elements alongwith heat treatment parameter has significant role to play in deciding the various end product purposes [14, 15]. The consensus has been that only a small amount of dissolved boron would be effective to increase the hardenability of steel and its effectiveness depends on the form of boron retained in the steel being influenced by the presence of other elements present in steel. In view of above, present work was carried out to understand effect of boron to nitrogen ratio and the different quenching media in low carbon low manganese unalloyed steel.
2. Experimental
- Tensile samples were prepared from the heats of composition with different B/N stochiometric ratio (shown in Table 1). These heats were industrially produced in LD converter and processed through ladle refining unit. Continuous cast slabs of 200 mm thickness were reheated and subjected to roughing and subsequently to finishing strands for achieving ~ 3 mm thickness in hot strip. Finishing temperature & coiling temperature maintained were 880°C and 570°C respectively. For comparative study, samples were also prepared from steel without boron, made and processed through same route.
|
2.1. Quenching of Low Carbon Boron Added Steel
- Tensile samples of hot rolled sheets were heated in laboratory muffle furnace at austenitising temperature (890°C) soaked and then quenched in oil and water medium. Mechanical properties and microstructure were analyzed of quenched samples.
2.2. Normalizing of Quenched Samples
- Quenched samples were further normalized at 920°C and properties were compared with as rolled boron free steel.
3. Results & Discussions
- There are two variables in this study to which material responded differently. These are boron content of steel and quenching media, i.e., cooling rate. Steel properties has also been further, analyzed and compared after normalizing with as rolled boron free steels to see the extent to which properties of steel regained.
3.1. Effect of Quenching on Low C Boron Added Steel
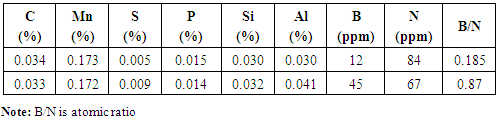
- Hardenability of boron-steel is affected by the precipitation behaviour of boron. In the work of Watanabe and Ohtani [16] with different kinds of precipitates of boron, which change their state depending on the heating temperature before rolling and quenching temperature during heat treatment of Al-B treated steels, they found that when the steels are furnace cooled (cooling rate of 0.67 C/min.) after hot rolling, AlN grows radially from BN which precipitated before the nucleation of AlN. AlN formed on BN during furnace cooling is dendritic. Al, B and N dissolve into solid solution during reheating of slab at 1300°C. On subsequent hot rolling and post cooling, aided by high diffusivity of B and N over Al, concentration of B and N atoms increases at grain boundaries to the extent that even solubility product is exceeded while Al atoms remain distributed homogeneously and do not prevent BN to precipitate. Thus, due to high diffusion velocity, BN precipitates on austenite grain boundaries. Under the situation, the effect of boron on the hardenability almost disappears. This situation corresponds to non equilibrium state of Al-B-N-Fe system. Habu et.al [17] showed by calculating B content in solid solution in the equilibrium state of Al-B-N-Fe system, that Al protects B from precipitating as BN by fixing N as AlN, which ensures presence of B in solid solution, needed for hardenability. The competitive precipitation of AlN and BN has been studied by several authors [18, 19]. It has been found that AlN can form at the expense of BN during equilibrium treatments in low alloy steels. In Figure 1a, UTS trend is first increasing as B/N ratio increased to 0.185 and then decreased at 0.87 B/N ratio for both media whereas, YS showing a downward trend as shown in Figure 1b. It is well known that YS is always governed by grain size whereas, UTS value depends upon factors like presence of secondary phases, distribution & size of phases, dislocation density, grain-size, etc. At lower B/N ratio i.e. 0.185, there plays role of higher AlN precipitates led finer grain size due to higher aluminium and absolute nitrogen, see Table 1 and also the available free boron which increased the hardenability. These two factors impart higher UTS value at 0.185 B/N ratio whereas, at higher B/N ratio (0.87), since there is higher concentration of B and lesser absolute nitrogen content, Al wasn’t found able to prevent B from precipitating as BN thereby, plays the combined effect of AlN and BN to reduce YS and UTS.
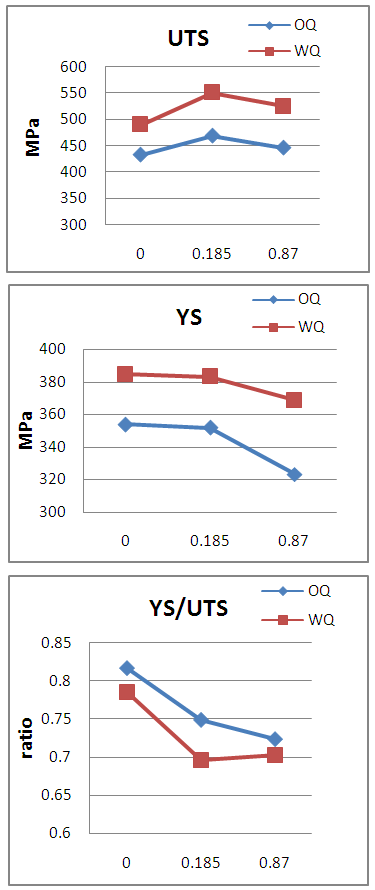 | Figure 1. Effect of quenching on mechanical properties of low C boron added steel (a) UTS (b) YS (c) YS/UTS |
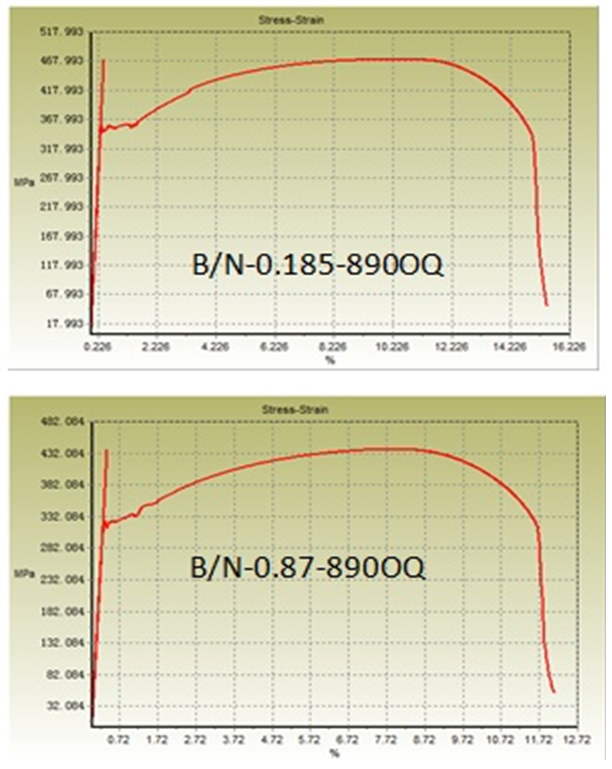 | Figure 2. Stress-strain curve of oil-quenched samples |
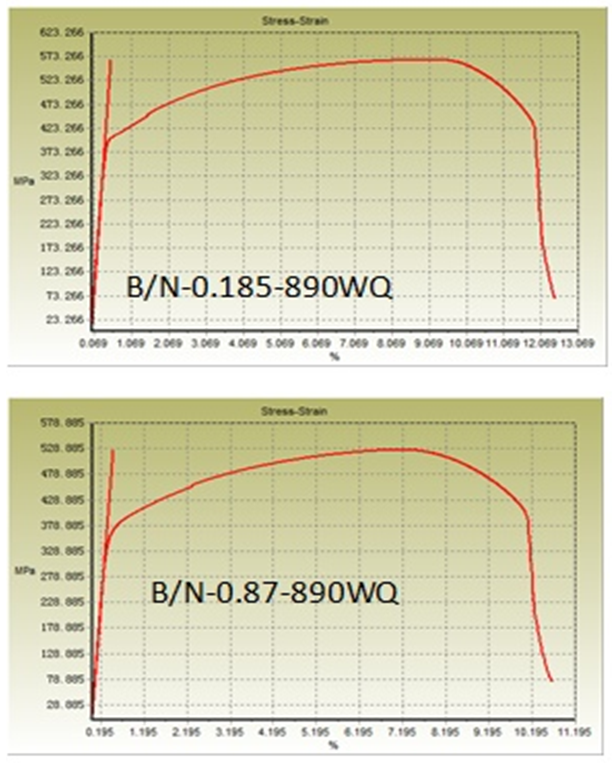 | Figure 3. Stress-strain curve of water-quenched samples. (Note: X-axis showing strain % based on elongation of total length of the sample.) |
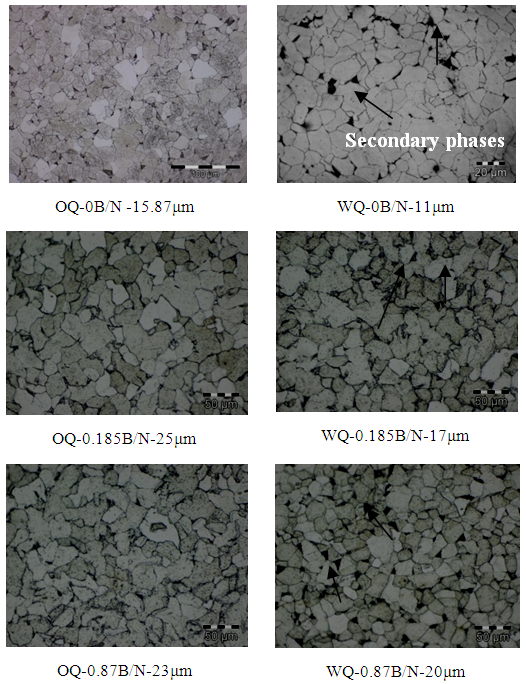 | Figure 4. Microstructure of quenched and normalized samples |
3.2. Effect of Normalizing of Quenched Samples
- From Figure 5a & b, it can be seen that normalizing improves properties in terms of YS and UTS for lower B/N ratio i.e., 0.185 for oil quenched samples but UTS increased similar to as rolled boron free steels for 0.87 B/N ratio water quenched steels. On the other hand, normalizing enabled lower YS/UTS value with boron addition to 0.87 B/N ratio. It varied from 0.74 to 0.65 in case of normalized oil quenched samples and to 0.69 in case of normalized water quenched samples. Although, with normalizing harder phases generally gets degenerated, but in some of conditions discussed earlier in this section, higher strength may be due to formation of contributing phases which gets retained in the microstructure even after normalizing [6-10]. Grain size effect should also be taken into account for the variation in yield strength as shown in Figure 6.
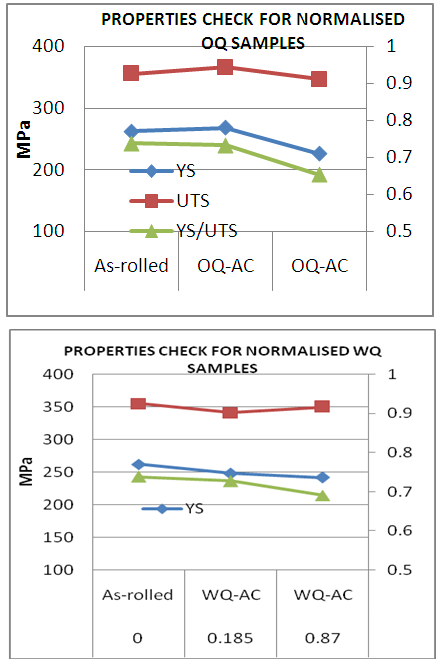 | Figure 5. Normalizing effect on (a) oil-quenched samples (b) water quenched samples |
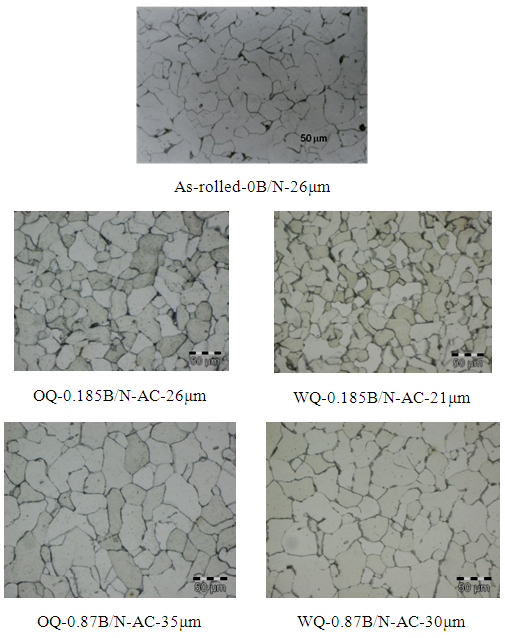 | Figure 6. Microstructure of normalized samples compared with as-rolled samples |
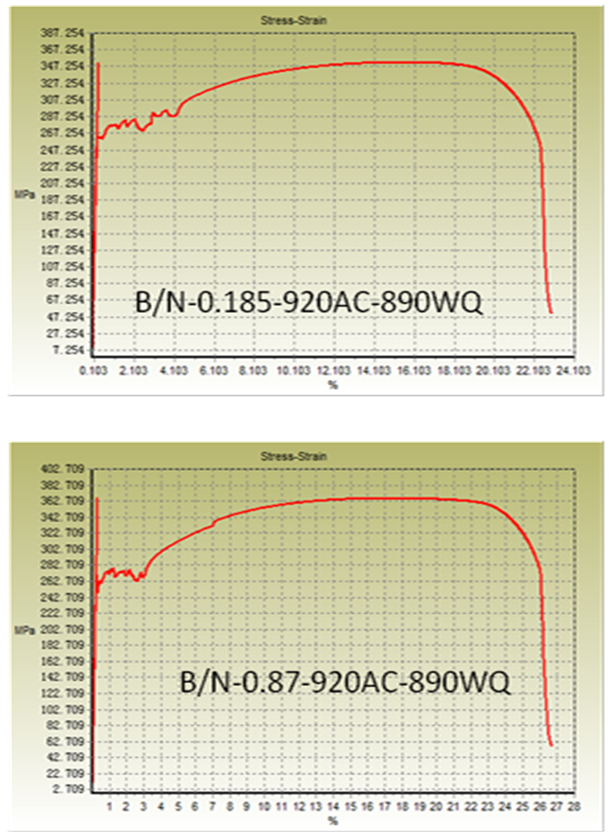 | Figure 7a. Stress-strain curve of normalized samples (water quenching) |
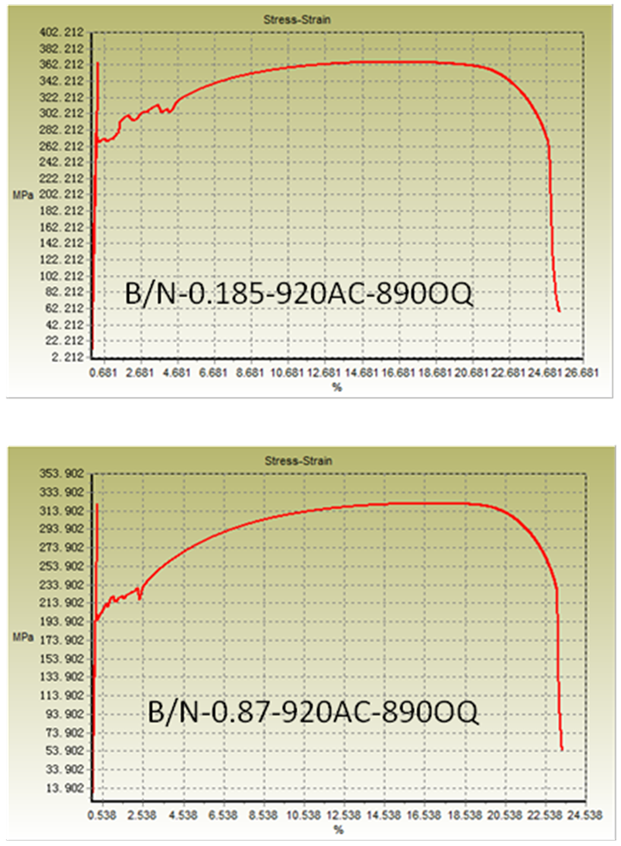 | Figure 7b. Stress-strain curve of normalized samples (oil quenched) |
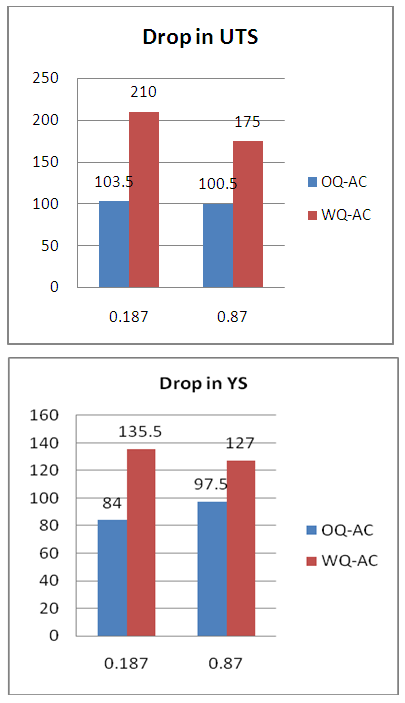 | Figure 8. Drop in strength after normalizing |
4. Conclusions
- a) It was observed that on water quenching YS varied from 385-369MPa, UTS from 490-525MPa and YS/UTS from 0.79 to 0.70 as the B/N ratio increased whereas in case of oil quenching, YS varied from 350-323MPa, UTS from 433-446MPa and YS/UTS from 0.82 to 0.72, respectively due to combined effect of boron as a hardenability agent, its content controlling the AlN precipitation in microstructure and the cooling rate which if faster causes continuous yielding.b) Good combination of properties observed in water quenched steel (YS-384MPa, UTS-551MPa, YS/UTS-0.69) with low B/N ratio (0.187) and oil quenched steel (YS-326MPa, UTS-447MPa, YS/UTS-0.73) with high B/N ratio (0.87). Also, with water quenching media, although lower YS/UTS value observed with boron addition, but it didn’t improve further as B/N ratio goes up from 0.185 to 0.87. c) Comparing further normalized quenched samples with as rolled boron free steel shows improvement in properties in terms of lower YS/UTS value with boron addition. It varied from 0.74 to 0.65 in case of normalized oil quenched samples and to 0.69 in case of normalized water quenched samples. Boron played an important role in contributing microstructure evolution with different quenching media and thereby resulted in better combination of properties in cost effective low carbon unalloyed steel.
ACKNOWLEDGEMENTS
- The authors would like to express appreciation for the support of Research and Development Centre for Iron & Steel, Ranchi for extending support for carrying out the research work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML