-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Metallurgical Engineering
p-ISSN: 2167-700X e-ISSN: 2167-7018
2013; 2(2): 243-248
doi:10.5923/j.ijmee.20130202.17
Extraction of Tin and Copper by Acid Leaching of PCBs
Amit Chaurasia, K. K. Singh, T. R. Mankhand
Department of Metallurgical Engineering, Indian Institute of Technology, (BHU), Varanasi, 221005, India
Correspondence to: Amit Chaurasia, Department of Metallurgical Engineering, Indian Institute of Technology, (BHU), Varanasi, 221005, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Printed circuit board (PCB) waste is generally dumped as a landfilling or treated pyrometallurgically, which consumes enormous power, generates hazardous gases and also destroys valuable PCB components. For recycling of the components, they must be separated from the printed circuit boards which is a cumbersome task. For its simplification, the rate of dissolution of solder with acids like Hydrochloric and Nitric of different concentrations was studied. Nitric acid was found suitable and used for the extraction of copper and tin at room temperature. In the leached solution suspended tin and copper nitrates were obtained. X-ray diffraction (XRD) and Energy dispersive X-ray analysis (EDAX) was done to characterize the precipitate obtained. Copper in leached solution was recovered by cementation.
Keywords: Electronic Waste, Printed Circuit Board, Solder, Leaching, Recovery
Cite this paper: Amit Chaurasia, K. K. Singh, T. R. Mankhand, Extraction of Tin and Copper by Acid Leaching of PCBs, International Journal of Metallurgical Engineering, Vol. 2 No. 2, 2013, pp. 243-248. doi: 10.5923/j.ijmee.20130202.17.
Article Outline
1. Introduction
- The production of electric and electronic equipment (EEE) is rapidly increasing in the world as a result of the revolution of information technology. Economic growth, technological innovation, market expansion and short life of EEE has lead to a significant increase in waste of EEEs (WEEE) which is an environmental threat[1][2]. About 50 million metric tonnes of E-waste is generated worldwide and is continuously increasing. Unfortunately, most developing countries have not introduced regulations in waste management[3].Many developing countries including India face the problem of hazardous e-waste generation with serious consequences for the environment and public health. According to the UN research in China and South Africa, e-waste from old computers may rise by 200 to 400 % from 2007 levels and by 500 % in India. In the same period, e-waste from mobile phones is predicted to rise 7 times in China, and by about 18 times in India. An estimated 3.8 lakh tonnes of e-waste is generated annually in India, of which only 19,000 tonnes are recycled. India faces a huge challenge to dispose of an estimated 4.2 lakh tonnes of e-waste a year that it generates domestically and by imports from abroad[4]. Ministry of environment and forest (MoEF) Govt. of India has notified E-waste Management Rules, 2011[5].WEEE is generally processed by feeding in a copper smelter or by landfilling. Landfilling can lead to contamination of water supplies by metals like lead and copper. The components (Capacitors, Resistors, IC’s etc), which have a lifespan of decades are destroyed in both the cases. For components to be removed, the solder which mainly comprises of tin and lead should be melted. But this high temperature can adversely affect the working of components. So an alternative approach is much needed which is focused on recovering the valuable components in working condition[6].Tin/lead solder (63% tin and 37% lead) was used as the most common solder alloy in electronics. Lead is highly toxic and raises several environmental concerns, including the disposal of the discarded electronic device containing lead. US Environmental Protection Agency (EPA) uses a method based on the toxicity characteristic leaching procedure (TCLP) to determine a solid waste’s status as a hazardous waste due to the toxicity characteristic (TC). Several studies have claimed that most of the times PCBs exceed the lead TC limit of 5 mg/L. Alternative solders are now a days gaining importance, including tin–copper and tin–silver–copper. The US EPA’s is promoting lead-free solders design while working with stakeholders. Additionally, the European Union Directive 2002/95/EC—Restriction of Hazardous Substances (RoHS) restricts the use of specific hazardous materials found in electrical and electronic products including lead. This will increase the use of alternative solders in the near future[7].Hydrometallurgy can offer an effective solution due to its flexible, environment-friendly and energy-saving features. A leachant can be developed that can selectively attack the solder while leaving the other parts unaffected. Components can be removed after solder dissolution and the remaining board can be treated with prior methods. Equally important aspects will be leachant regeneration and the extraction of valuable metals like tin and copper from the leachant.Metal extraction with nitric acid have been investigated by Cerna (1992), Zongcheng (1989)[8][9]. More than 95 % copper and lead extractions were achieved by Mecucci and Scott (2002) using nitric acid[10]. Purification of nitric acid leach solutions was studied by Le (2011)[11], Kinoshita (2003)[12], Man-Seung Lee (2003)[13], Brown, 1980[14], Mockrin and Hobin, 1977[15], Nelson, 1977[16] and found that the reagent can be recovered effectively.Buckle and Roy investigated thermodynamic analysis of copper–nitrate–water system and tin–nitrate–water system [17].The E-pH diagram for tin–nitrate–water system as shown in Fig. 1 is independent of the concentration of nitrate. Tin exists as a dissolved species across a small range of potentials at around 0V, or if the pH is less than -0.42 in the acidic region as shown in the diagram. If pH is greater than 12.6 in very alkaline conditions tin is found to exist as a dissolved species. For the remaining pH range (-0.42 < pH< 12.6), the tin exists as the solid stannous oxide.
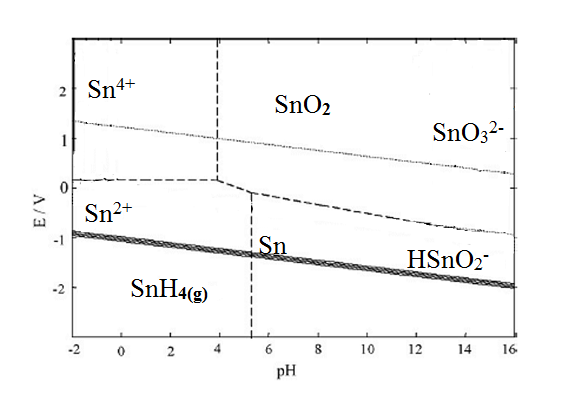 | Figure 1. E–pH diagram for tin–nitrate–water (298 K, aNO3= 1) |
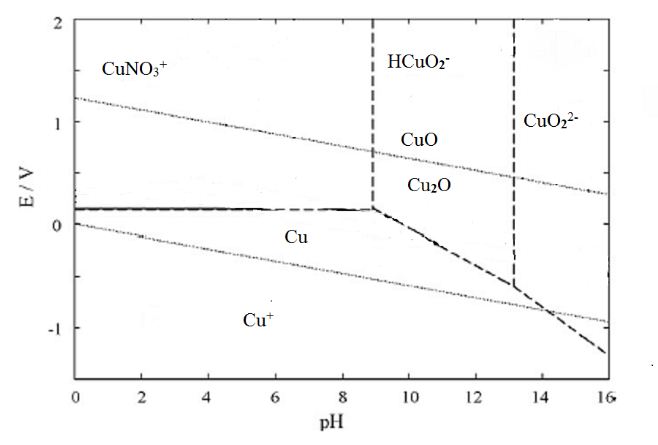 | Figure 2. E–pH diagram for copper–nitrate–water system (298 K, aNO3=1) |
2. Materials and Methods
2.1. Dissolution Studies
- Waste PCBs were first cut into small pieces of about 3 cm×3 cm by pliers. Acid baths of nitric and hydrochloric acids of known concentration were made and PCB parts were immersed for desired time at room temperature. For the study of solder dissolution, acid concentrations were kept low so that the epoxy layer does not react with the acid and only solder dissolution takes place. Then, the components were taken out at intervals of 15 minutes, washed, dried and weighed for determining the amount of solder dissolved.For determining the time required for complete dissolution of solder, parts were immersed in acids of known concentration and time required was noted when components could be removed by shaking.
2.2. Recovery of Tin and Copper
- For recovering tin and copper, a solution of 18 % Nitric acid was taken and PCB pieces weighing 500 grams were dissolved in it for 2 hours till all solder was dissolved. PCB components were removed and solution was filtered with an ashless filter paper to get copper nitrate in the solution and stannic acid in the precipitate. It was heated in a muffle furnace at 600ºC for an hour to get stannic oxide. The resulting solid was found to be 34.6 grams. X-ray diffraction (XRD) and Energy dispersive X-ray analysis (EDAX) was done for its characterization.For copper in the leached solution, sulfate bath was made by adding few drops of sulfuric acid and evaporating all the nitric acid. It was titrated to get the amount of copper present. For recovery, Cementation was carried out at 60 °C till all copper was recovered.
3. Results and Discussion
- Rate of solder dissolution with time for different acid concentrations was studied. Sulfuric acid was found unable to leach the solder at low concentrations, so further study was discarded. Perchloric acid was also found ineffective.Nitric acid, a strong oxidizing agent has selective dissolution properties for copper, tin and lead. Moreover, it is cheap and the possibility of its regeneration makes it preferable to HCl which may form unwanted precipitates. It forms a precipitate of metastannic acid on reaction with tin. With copper and lead, it forms soluble nitrates[10].3Cu + 8HNO3 = 3Cu(NO3) 2 + 4H2O + 2NOPb + 2HNO3 = Pb(NO3) 2 + H2Sn + 4HNO3 = H2SnO3 ↓ + 4NO2 + H2OOn heating, metastannic acid converts to stannic oxide.H2SnO3 + Heat = SnO2 + H2OIt was observed that reaction was almost instant when the concentration of nitric acid was above 50 %. At low concentrations, the reaction rates were reduced drastically. Time required for 25 %, 20 %, 18 % and 15% acid concentrations were 60, 90, 105 and 120 minutes respectively. Hydrochloric acid of 36 % concentration required an hour while for lower concentrations, reaction rates were reduced drastically. It is evident from the fact that rate of reaction is directly proportional to concentration gradient.Fig. 3 and 4 shows the dissolution of solder in nitric and hydrochloric acids respectively. Dependence of dissolution time of solder on acid concentration is shown in Fig. 5
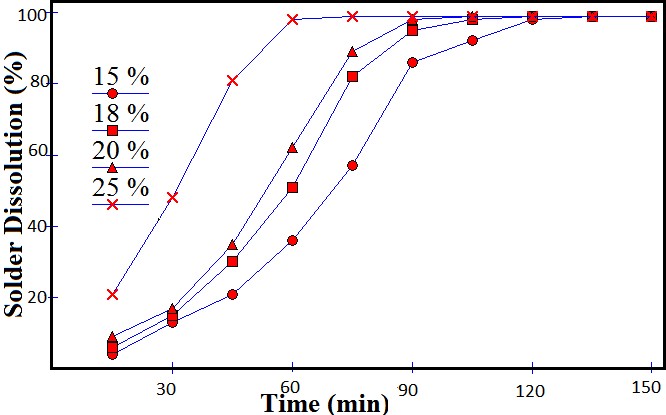 | Figure 3. Dissolution of solder in Nitric acid |
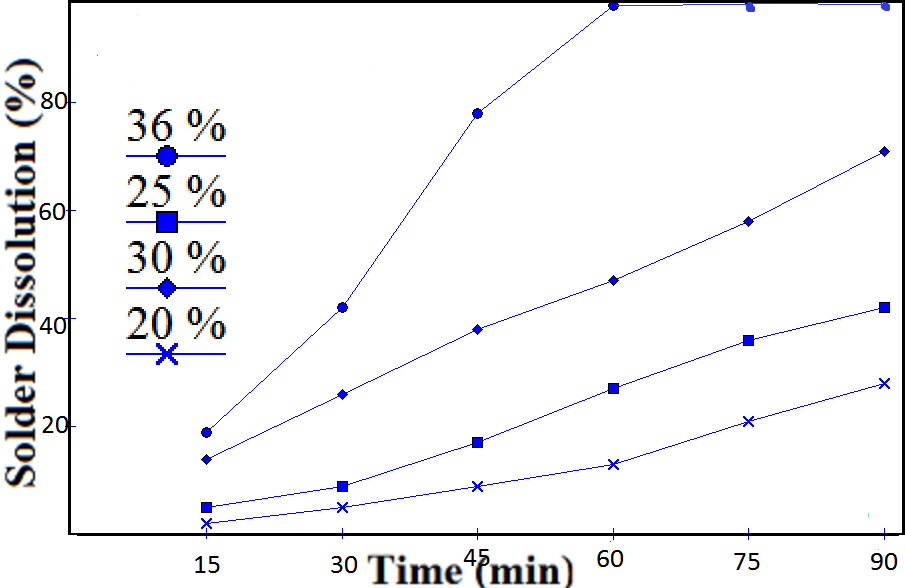 | Figure 4. Dissolution of solder in Hydrochloric acid |
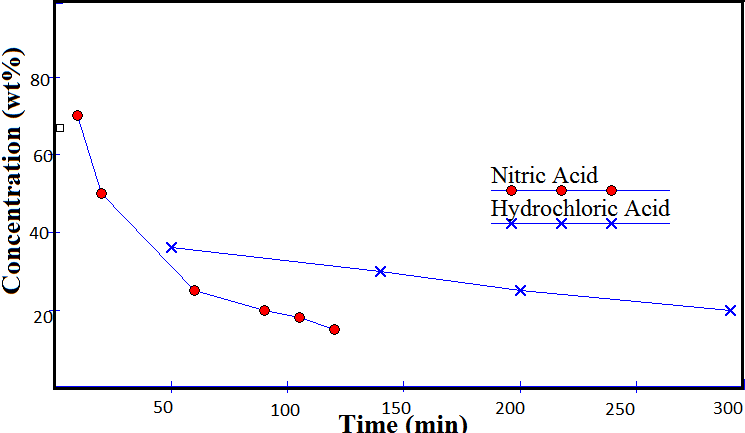 | Figure 5. Plot of time of dissolution vs acid concentration (weight %) |
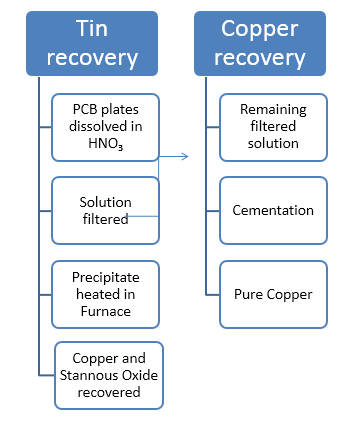 | Figure 6. Schematic representation of recovery of Sn and Cu from PCB’s |
 | Figure 7. EDAX analysis of precipitate |
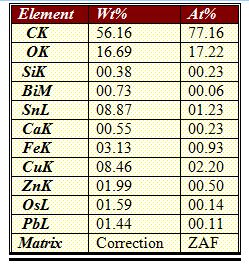 | Figure 8. Elemental Constitution of precipitate by EDAX |
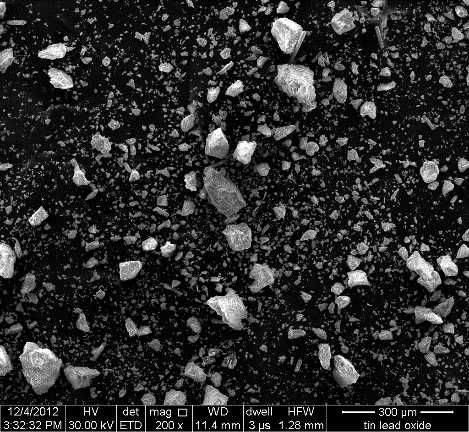 | Figure 9. Scanning electron microscope image of precipitate particles at 200X |
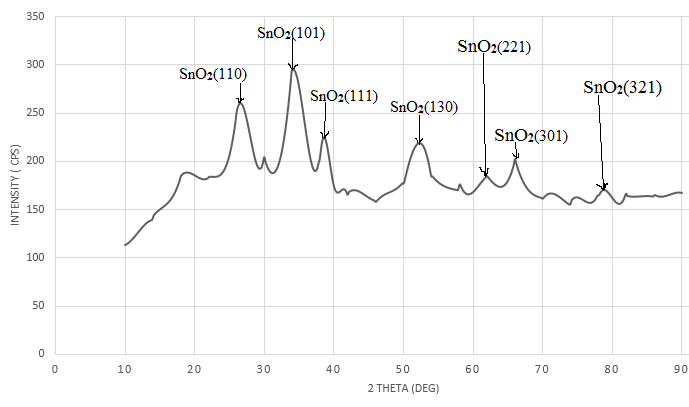 | Figure 10. XRD analysis of the precipitate |
4. Conclusions
- The leaching time increases drastically when the concentration of nitric acid was reduced. For greater than 50% concentrated acid, reaction was instant and finished within 15 minutes. Whereas for less than 20 % acid concentration, it took about two hours for the reaction to complete.Tin which comprises more than 50 % of the solder can be recovered easily as stannic oxide with hydrometallurgical route alone. Per 100 grams of PCB plates 6.92 grams of precipitate was recovered which contains primarily of oxides of copper and tin.Copper was recovered by cementation from a solution of copper sulfate with 73.5 % efficiency.1.14 grams of copper was recovered from leached solution obtained from 500 grams of PCB plates.PCB Components were not destroyed at low acid concentrations and on checking with a multimeter were found to be working
ACKNOWLEDGEMENTS
- The authors are grateful to the valuable services provided by the Head of Department, Metallurgical Engineering, Indian Institute of Technology, Banaras Hindu University.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML