-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Metallurgical Engineering
p-ISSN: 2167-700X e-ISSN: 2167-7018
2013; 2(2): 149-153
doi:10.5923/j.ijmee.20130202.06
Microstructural Evolution and Mechanical Properties of Type 304 L Stainless Steel Processed in Semi-Solid State
Dipti Samantaray1, Vinod Kumar2, A. K. Bhaduri1, Pradip Dutta3
1Materials Development and Technology Group, IGCAR, Kalpakkam, 603102, India
2Steel Products Group, R&D Centre for Iron & Steel, SAIL, Ranchi, 834002, India
3Department of Mechanical Engineering, Indian Institute of Science, Bangalore, 560012, India
Correspondence to: Dipti Samantaray, Materials Development and Technology Group, IGCAR, Kalpakkam, 603102, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The paper discusses the effect of semi-solid processing on the microstructural evolution and mechanical property of 304L stainless steel. For the study, steel specimens were partially melted and cooled to room temperatures in different cooling medium. The effect of temperature, time and cooling medium on microstructural evolution was studied by using optical microscopy. It was found that melting begins with the nucleation of liquid phase at the triple junctions and grain boundaries followed by propagation of the liquid phase along grain boundaries. The mechanical behavior of the semi-solid processed material was compared with that of the conventionally processed material with regard to their tensile properties and hardness. The semi-solid processed material shows better ductility and reduced YS and UTS than the conventionally processed counterpart. The correlation of the tensile properties and evolved liquid content shows that the UTS of the material decreases, while the YS increases with liquid fraction. The study also shows that there is a significant effect of cooling medium on the microstructural evolution and hence on the mechanical properties.
Keywords: 304L Stainless Steel, Semi-Solid Processing, Microstructure, Tensile Properties, Hardness
Cite this paper: Dipti Samantaray, Vinod Kumar, A. K. Bhaduri, Pradip Dutta, Microstructural Evolution and Mechanical Properties of Type 304 L Stainless Steel Processed in Semi-Solid State, International Journal of Metallurgical Engineering, Vol. 2 No. 2, 2013, pp. 149-153. doi: 10.5923/j.ijmee.20130202.06.
Article Outline
1. Introduction
- AISI type 304L austenitic stainless steel (304L SS) is widely used in power, chemical, petrochemical[1] and nuclear industries[2]. Conventionally, stainless steels are fabricated by casting and forging processes. However, continuous efforts are being made to find alternatives manufacturing routes to accomplish many objectives such as reducing energy consumption, enhancing workability, producing near-net shape products, and minimizing intermediate process steps[3]. Semi-solid processing is one such promising alternative to conventional manufacturing techniques[4], which not only fulfills the above objectives but also combines the benefits of both conventional casting and forging processes. This technology has already been adopted in an industrial scale for manufacturing of various non-ferrous alloys[4], such as aluminum[5] and magnesium alloys[6, 7]. The suitability of this technology has also been investigated for producing some components in laboratory scale from the ferrous alloys, such as X210Cr[3, 8] and bearing steels[9]. Therefore, adoption of this technology for other grades of steels has drawn worldwide attention. The major constraints in semi-solid processing of steels arise from high melting point of these alloys, narrow semi-solid temperature range, and phase transformation during melting and solidification[10]. In addition to the processing-related issues, assessing the suitability for semi-solid processing of steels also requires evaluation of post-processing mechanical properties. Therefore, the present work focuses on the microstructural evolution in 304L SS after cooling from the semi-solid state and on the resultant mechanical properties. A comparative study of the microstructure and mechanical properties of semi-solid and conventionally processed 304L SS is presented and discussed.
2. Experimental
- For the present study, commercially available solution annealed 304L SS plates, with chemical composition Fe-18.52Cr-7.85Ni-0.016C-1.59Mn-0.29Si-0.003S-0.016P, have been used.The microstructure of the steel in as-received condition is shown in Figure1. Small bars of size 15mm × 15mm × 120mm were cut from the plates and used to carry out the experiments in the semi-solid state. The bars were partially melted in a muffle furnace and then allowed to cool to room temperature inside the furnace. To investigate the effect of higher heating and cooling rates on the microstructural features of the material, a few cylindrical specimens of size of 10 mm diameter and 15 mm height were heated in a Gleeble thermo-mechanical simulator to the semi-solid temperature, held at the targeted temperature for 30s, and then quenched in water. The experimental details are given in Table.1 After cooling the bars and cylinders to room temperature, a small sample of each specimen was taken for microstructural investigation. The diamond polished metallographic samples were etched in 10% oxalic acid solution at 2V, and microstructures were observed using an optical microscope.
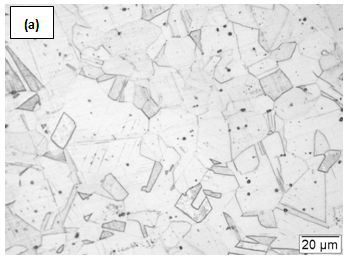 | Figure 1. Microstructure of solution annealed SS 304LTable 1: Parameters used for heat treatment of SS 304L |
|
3. Results and Discussions
3.1. Microstructural Evolution
- Microstructural evolution of any material depends on its chemical composition[11] and various parameters defined by the processing route such as heating rate, temperature of processing[12], cooling rate[13], mechanical loading[12], and so on. In any investigation on the effect of the processing route or the parameters involved in the process on the microstructure, it is always necessary to understand the effect of composition, as it defines the various phases that form within the processing temperature regime. In semi-solid processing, the material is heated to a temperature between the solidus and liquidus temperatures and then cooled to room temperature. Hence, it is of paramount interest to know the possible phases that can form between room temperature and the liquidus temperature. It is well known that the 304L SS retains its austenite phase at room temperature and undergoes phase changes at high temperatures, depending on the Cr-equivalent (Creq) to Ni-equivalent (Nieq) ratio of the steel[14]. In order to calculate the Creq and Nieq for different grades of 304 SS, the following equations are used[14]:
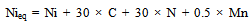 | (1) |
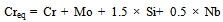 | (2) |
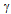 ) completely transforms to delta-ferrite
) completely transforms to delta-ferrite 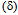 and further heating causes melting. The transformation follows the reverse order during cooling. At the solidus temperature, the liquid phase is nucleated at triple junctions and grain boundaries depending on the solid-liquid interface energy (
and further heating causes melting. The transformation follows the reverse order during cooling. At the solidus temperature, the liquid phase is nucleated at triple junctions and grain boundaries depending on the solid-liquid interface energy (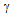 SL) and the grain boundary energy (
SL) and the grain boundary energy (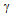 GB)[15]. With further increase in temperature, the liquid phase propagates along the grain boundaries and forms a continuous network along the grain edges. The growth of liquid phase along the grain edges causes a continuous decrease in size of the grains which gradually approach a critical size. Once the grains achieve the critical size, rapid melting occurs and the grains disappear in the molten metal pool[16]. However, in this study, the material has not been allowed to undergo complete melting. The material has only been allowed to melt partially, after which it was cooled to room temperature. The microstructures of samples that were furnace-cooled after partial melting (Figure 2) indicate the solidified fraction of the liquid around the grains.
GB)[15]. With further increase in temperature, the liquid phase propagates along the grain boundaries and forms a continuous network along the grain edges. The growth of liquid phase along the grain edges causes a continuous decrease in size of the grains which gradually approach a critical size. Once the grains achieve the critical size, rapid melting occurs and the grains disappear in the molten metal pool[16]. However, in this study, the material has not been allowed to undergo complete melting. The material has only been allowed to melt partially, after which it was cooled to room temperature. The microstructures of samples that were furnace-cooled after partial melting (Figure 2) indicate the solidified fraction of the liquid around the grains.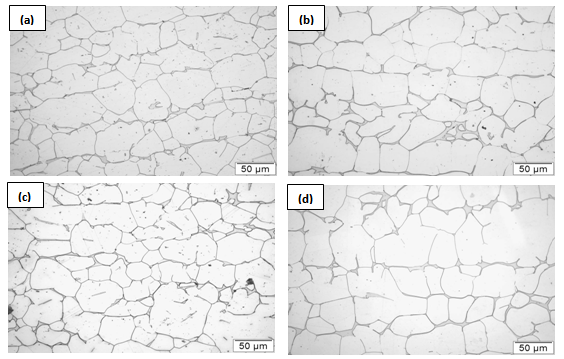 | Figure 2. Microstructural evolution in 304L due to partially melting at (a) 1400°C/5 mins (b)1410°C/ 5min (c) 1420°C /5min (d) 1400°C/ 25 min followed by furnace cooling |
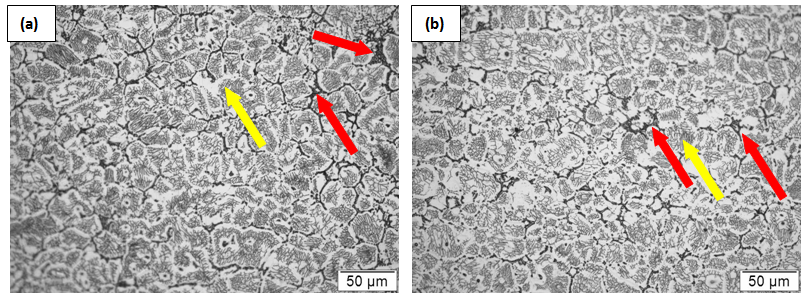 | Figure 3. Microstructural evolution in 304L due to partially melting at (a) 1410°C (b)1420°C followed by water quenching |
 transformation due to high cooling rate (
transformation due to high cooling rate ( 1500ºC/s) obtained on water quenching by spray nozzles in the Gleeble thermo-mechanical simulator. These delta-ferrite networks are not visible in the microstructures of the furnace-cooled samples, as in this case, the material does get sufficient time for
1500ºC/s) obtained on water quenching by spray nozzles in the Gleeble thermo-mechanical simulator. These delta-ferrite networks are not visible in the microstructures of the furnace-cooled samples, as in this case, the material does get sufficient time for  transformation.
transformation.3.2. Mechanical Properties
- Tension test is widely used to provide the important information on the strength of a material for the design of the product or for making structures using the material[18]. Therefore, to study the effect of the process parameters as well as the microstructure on the strength, it is required to evaluate the tensile properties of the material. However, it is always not possible to measure the tensile properties of a small volume of material produced in a laboratory scale. Therefore, hardness testing provides an alternative means of assessing their mechanical properties[18]. There are several relationships available to correlate the hardness with the yield strength and ultimate tensile strength of materials[18, 19]. In this investigation, both tensile properties and hardness have been evaluated for the samples that were furnace-cooled after partial melting, while only the hardness has been determined for the samples that were water-quenched after partial melting, due to size limitation of the samples used. The yield strength (YS), tensile strength (UTS) and the ductility (percent uniform elongation) obtained from the tensile tests performed on the as-received material (conventionally processed) and on the semi-solid processed material (with furnace- cooling) are given in Table 2. From the table, it is observed that the material processed in the semi-solid range shows better ductility than the conventionally processed (by hot rolling followed by solution annealing) material, i.e. However, these materials show reduced YS and UTS compared to the conventionally processed one. Similar comparison of the tensile properties of a cast product of 304L SS reported in literature[20] shows that the semi-solid processed material shows better UTS and ductility with a comparable YS value. The correlation between the fraction of liquid and the tensile properties are shown in Figure4. From this figure an overall decrease in UTS is observed over the entire domain of study, while the YS is found to have improved with increase in liquid content. On the other hand, an initial decrease in the ductility is observed up to a liquid fraction of 14% beyond which the ductility increases with increase in liquid fraction. Here, it is to be noted that the liquid fraction in the specimens have been calculated using image analysis technique. For calculation of the liquid fraction in a particular specimen, an average of 15 micrographs of each specimen has been used. The micrographs have been taken from various locations of the specimen at different magnifications. The errors involved in the calculation could be minimized by carrying out thermal analysis of the material in the solidus–liquidus temperature domain. Table 2 also shows hardness values of these materials. The hardness values of the furnace-cooled material are found to be lower than those of the as-received material; while these are found to be similar to the hardness value of the cast steel. The variation of hardness with the liquid fraction shows a similar trend as that of the YS. These results agree with the earlier findings on the relationship between the hardness and YS of austenitic steels[19].
|
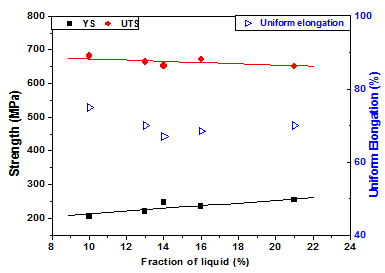 | Figure 4. Correlation between the fraction of liquid and the tensile properties |
4. Conclusions
- The effect of semi-solid processing on the microstructural evolution and mechanical properties of a type 304L SS has been studied. Towards this end, the partially 304L SS were cooled to room temperature using different cooling medium. Tensile properties and hardness of the specimens were evaluated to compare the mechanical behaviour of the semi-solid processed material with the conventionally processed material. Also, the effect of evolved liquid content during semi-solid processing on the mechanical properties of this material has been studied. The following conclusions can be drawn from the above investigation.1. The solidification mode of type 304L SS is primary ferrite. With increase in temperature, severe grain growth occurs in the specimen, and melting begins with the nucleation of liquid phase at the triple junctions and grain boundaries followed by propagation of the liquid phase along the grain boundaries.2. An increase in melting temperature causes the nucleation of liquid phase at the grain interiors along with the triple junctions and grain boundaries, whereas increase in soaking time causes growth of the nucleated phase.3. The semi-solid processed material shows better ductility and reduced YS and UTS than their conventionally processed counterpart that was solution annealed after hot rolling.4. An overall decrease of UTS in observed over the entire domain of study; the YS is found to improve with the evolved liquid content.5. Hardness of water quenched specimens is superior to that of furnace cooled specimens. The superior resistance to plastic deformation has been attributed to the presence of fine delta-ferrite networks in the water quenched specimens.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML