-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Metallurgical Engineering
p-ISSN: 2167-700X e-ISSN: 2167-7018
2012; 1(6): 102-107
doi: 10.5923/j.ijmee.20120106.01
Pyrolysis of Printed Circuit Boards
T. R. Mankhand, K. K. Singh, Sumit Kumar Gupta, Somnath Das
Department of Metallurgical Engineering, Indian Institute of Technology (Banaras Hindu University),Varanasi, 221005, India
Correspondence to: T. R. Mankhand, Department of Metallurgical Engineering, Indian Institute of Technology (Banaras Hindu University),Varanasi, 221005, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Printed Circuit Board (PCB) is an essential component of almost all electrical and electronic equipments. The rapid growth of the use of such equipments has contributed enormously to the generation of large quantity of waste PCBs. The WPCBs not only contain valuable metals but also a large variety of hazardous materials. Conventional treatments of such WPCBs have their own limitations. By pyrolysis of WPCBs, it is not only possible to obtain the organic part of it as a fuel or useful chemical but can make further processing to recover metals much easier and efficient. In the present work, a kinetic study on the low temperature pyrolysis of WPCBs using a thermogravimetric analyser has been attempted. The TG analysis was conducted in nitrogen and air atmospheres in the temperature range of 200-600℃ at a heating rate of 40℃/min. The kinetic expressions for both the environments were determined and the activation energies were found to be 110.7 and 90.2 kJ/mol for nitrogen and air, respectively. The effect of thermal pre-treatment on the subsequent copper leaching in nitric acid for untreated, pyrolysed and air-burned PCB was also studied. Copper recoveries from these samples were 30.4%, 92.5% and 96.2%, respectively indicating the importance of thermal pre-treatment in leaching of the metal content.
Keywords: Pyrolysis, Printed Circuit Board (PCB), E-waste, Copper leaching
Cite this paper: T. R. Mankhand, K. K. Singh, Sumit Kumar Gupta, Somnath Das, "Pyrolysis of Printed Circuit Boards", International Journal of Metallurgical Engineering, Vol. 1 No. 6, 2012, pp. 102-107. doi: 10.5923/j.ijmee.20120106.01.
Article Outline
1. Introduction
- Printed Circuit Board (PCB) is an essential component of almost all electronic and electrical equipments such as computers, televisions, mobile phones, entertainment devices, household appliances and other such items. The rapid growth of the use of such equipments, combined with their early obsolescence has contributed enormously to the generation of large quantity of electronic waste (e-waste). The UNEP estimates that the world is generating collectively about 20-50 million tons of e-waste every year.It is also predicted that by the year 2020, e-waste in India from old computers will jump 5 times, while from discarded mobile phones will be 18 times higher compared to 2007 level.The printed circuit board is a major constituent of the obsolete and discarded electronic scrap and it accounts for approximately 30% of the total e-scrap generated. It has homogenous mixture of organic material, metals and glass fiber. The non-metals such as epoxy, glass fibre and other additives constitute about 70% by weight of PCBs while remaining 30% constitute metals such as copper, tin, lead, iron and nickel. In the metal fraction the approximate contents are: copper-17%, solder-4%, iron-3% and nickel-2%. Precious metals such as gold, silver and palladium are also present in small quantities[1-2]. PCBs also contain a variety of heavy metals and hazardous substances viz., lead, cadmium, mercury, PVC and halogenated flame retardants etc. that may seriously pollute the environment if they are not properly disposed of. Moreover, the recycling of WPCBs is difficult due to their multi-component and multi-layered construction. Therefore, developing a non-polluting and economical processing technology for recycling of WPCBs is needed not only to recycle valuable resources but also to avoid environmental pollution.Traditionally, pyrometallurgical[3], hydrometallurgical [4-6] and mechanical processing[7-9] have been used to recycle WPCBs. Pyrometallurgical processes involve combustion, smelting in molten bath etc. In this process crushed scrap is charged in a molten bath to remove plastics and refractory oxides to form a slag containing valuable metals. However, it leads to formation of hazardous by-product fumes and only a partial recovery of metals could be achieved. Compared to pyrometallurgy, hydrometallurgical methods are more exact, highly predictable and easily controlled but many of the solvents used like cyanides and chlorides are highly hazardous. It also involves large number of steps, has high cost of recovery and generates waste solutions and sludge which create environmental pollution. Mechanical processingincludes multi-crushing, grinding, gravity, electrostatic, magnetic and density based separation methods. However, such methods produce much dust and harmful particulate matter. Moreover, the non-metallic powder obtained can only be used as low-value products, viz. paint, paving material, plastic filling material etc., while the precious metals may be lost as they usuallyadhere to the non-metallic powder in crushing and grinding.Pyrolysis is a thermochemical decomposition of organic material at elevated temperatures (generally without the participation of oxygen). It involves the simultaneous change of chemical composition and physical phase, and is irreversible. It can be considered as an alternative method for recycling PCB, because in the pyrolysis process, the organic part is decomposed to low molecular products (liquids or gases), which can be used as fuel or chemical source, furthermore PCB changes brittle, undergoes delamination which could be easily crushed, while the inorganic part such as glass-fibre remain fairly intense, which can be recycled into other composites or any other materials[10-11].Pyrolysis is a thermal recycling technique that has been widely researched as a method of recycling synthetic polymers including polymers that are mixed with glass fibres. Although a significant amount of research into the pyrolysis of PCBs has been reported, most of the work has been carried out under nitrogen atmosphere using analytical pyrolysis techniques or laboratory scale reactors involving measurement of kinetics[12-15] and characterization of its products obtained[16-18]. In this present study a kinetic analysis of the low temperature pyrolysis of WPCBs has been studied under nitrogen and air atmosphere. The effect of thermal pre-treatment on the leaching of copper from untreated, pyrolysed and air-burned (combustion) samples was also examined.
2. Experimental
2.1. Materials
- Printed Circuit Boards (PCBs), (a part of computer mother board) were collected from old and obsolete computers through local sources (Figure.1 (a)). The batteries, capacitors and other electronic devises from PCBs were mechanically removed in order to get clean and component free PCBs. A number of such boards were crushed using laboratory jaw crusher to get pieces in the size range of 3-5 cm. These large pieces were further reduced to size of 3-5 mm (Figure.1 (b)) using a manual cutter.
 | Figure 1. (a)PCB from computer mother board (b) after cutting |
2.2. Apparatus and Methods
2.2.1. Pyrolysis
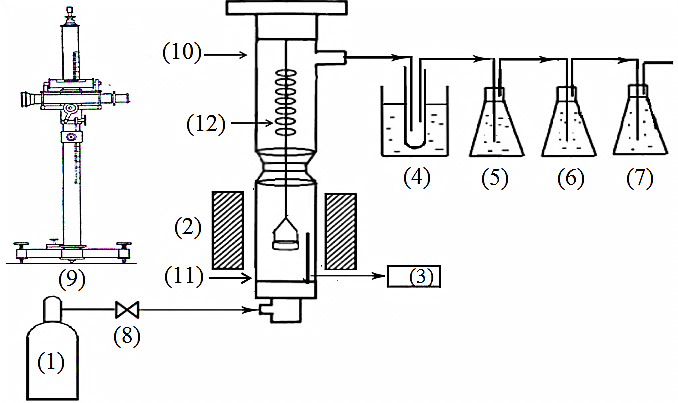
- The PCB particles of the size in the range of 3-5mm were subjected to pyrolysis under nitrogen atmosphere using a thermogravimetricanalyser. It measures continually the change in weight of the samples undergoing reactions at the specified temperatures with the help of a cathetometer. A schematic diagram of the apparatus is shown Figure. 2.
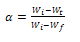 | (1) |
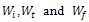 , represents initial, at time t and final weight loss of the sample, respectively. The pyrolysis experiments were carried out in the temperature range of 200-600℃ at an interval of 100℃ and at a heating rate of 40℃/min, in each case until the set temperature was obtained. Upon reaching the requisite temperature it was maintained constant throughout the entire process. When the experiments were finished, the furnace power was turned off but the carrier gas was kept flowing until the reactor was cooled down to the room temperature. High purity nitrogen was used as a purge gas and the exiting products of pyrolysis were trapped in the condenser system as shown in Figure 2. It consisted of a water cooled empty condenser, distilled water to trap the water soluble products and the remaining two containing 1M H2SO4 and 1M NaOH solutions to take care of other products of pyrolysis. In order to observe the effect of environment on pyrolysis behaviour separate experiments were conducted in air under similar conditions. However no condensation system was employed during these experiments.
, represents initial, at time t and final weight loss of the sample, respectively. The pyrolysis experiments were carried out in the temperature range of 200-600℃ at an interval of 100℃ and at a heating rate of 40℃/min, in each case until the set temperature was obtained. Upon reaching the requisite temperature it was maintained constant throughout the entire process. When the experiments were finished, the furnace power was turned off but the carrier gas was kept flowing until the reactor was cooled down to the room temperature. High purity nitrogen was used as a purge gas and the exiting products of pyrolysis were trapped in the condenser system as shown in Figure 2. It consisted of a water cooled empty condenser, distilled water to trap the water soluble products and the remaining two containing 1M H2SO4 and 1M NaOH solutions to take care of other products of pyrolysis. In order to observe the effect of environment on pyrolysis behaviour separate experiments were conducted in air under similar conditions. However no condensation system was employed during these experiments.2.2.2. Leaching
- Leaching of the pyrolysed samples were carried out to assess the recovery of copper. It was done in 1M solution of HNO3 at 80℃ for 60 minutes using constant stirring in a glass reactor. In order to compare the leaching behaviour, the samples without thermal treatment, the ones burned in air and the ones pyrolysed in nitrogen were leached under similar conditions. The leaching experiments were carried out in a Pyrex beaker provided with a heater to maintain the requisite temperature. The pulp densities used in all the cases were 25g/L. All the samples had retained their shape and size. A stirring speed of 300 rpm was maintained to avoid splashing of acid. During experiments, the liquid samples were withdrawn at regular time intervals and analysed for their copper content using a spectrophotometer.
3. Results and Discussion
3.1. Thermogravimetric Results
3.1.1. In Nitrogen
- To evaluate the rate of pyrolysis of PCB particles, thermogravimetric studies were conducted at various temperatures viz. 300, 400, 500, 600℃ at a constant heating rate of 40℃/min. under nitrogen atmosphere. The results are shown in Figure. 3. The percentage weight loss of the samples was calculated at various time intervals at different temperatures of study.
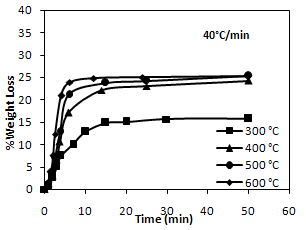 | Figure 3. TG curves of pyrolysis of PCBs in nitrogen atmosphere |
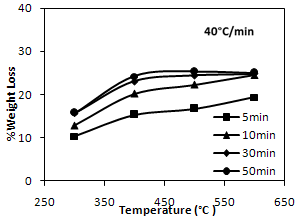 | Figure 4. Temperature dependence of weight loss under nitrogen atmosphere |
3.1.2. In Air
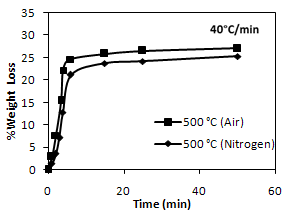 | Figure 5. Comparison of pyrolysis performances of PCBs under nitrogen and air |
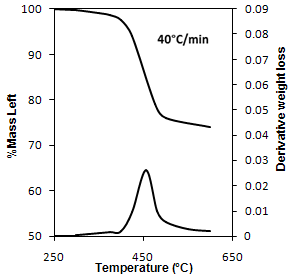 | Figure 6. TG and DTG curves depicting stages of pyrolysis under nitrogen atmosphere |
3.3. Pyrolysis Kinetics
- It is possible to describe the pyrolysis behaviour of PCB scraps under different conditions by different pyrolysis mechanisms[13],[19-22]. The decomposition rate of the pyrolysis process depends on an arbitrary reaction order. The reaction scheme can be represented as:
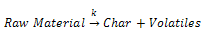 Where k is the rate constant of the reaction following the Arrhenius law. It was assumed that the total reaction accords with the following conventional equation:
Where k is the rate constant of the reaction following the Arrhenius law. It was assumed that the total reaction accords with the following conventional equation: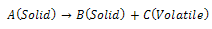 | (2) |
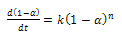 | (3) |
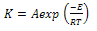 | (4) |
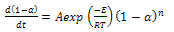 | (5) |
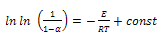 | (6) |
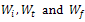 , represents initial, at time t and final weight loss of the sample, respectively. Based on the information from the TG analysis and the above equations the
, represents initial, at time t and final weight loss of the sample, respectively. Based on the information from the TG analysis and the above equations the 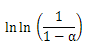 value along with
value along with  under air and nitrogen atmospheres were calculated respectively, and are plotted, as shown in Figure. 7(a) and (b), respectively.The apparent activation energies were found to be 90.26 and 110.7 kJ/mol for the atmospheres of air and nitrogen surroundings, respectively.The corresponding kinetic equations for these atmospheres are:
under air and nitrogen atmospheres were calculated respectively, and are plotted, as shown in Figure. 7(a) and (b), respectively.The apparent activation energies were found to be 90.26 and 110.7 kJ/mol for the atmospheres of air and nitrogen surroundings, respectively.The corresponding kinetic equations for these atmospheres are: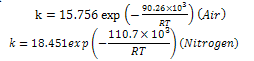 | (7) |
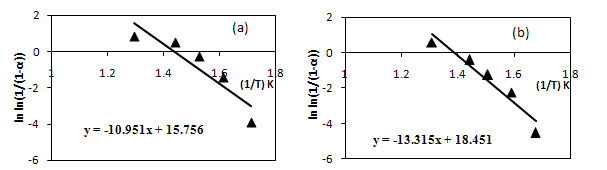 | Figure 7. Kinetics expressions under (a) Air (b) Nitrogen atmosphere |
3.4. Pyrolysis Yield
- In the case of burning, the copper particles were covered with oxides. While the samples were partly carbonised due to the pyrolysis, and they differed from the samples exposed to the burning due to its dark colour. The copper particles during pyrolysis remained unreacted. Figure. 8 represents the difference between these samples.
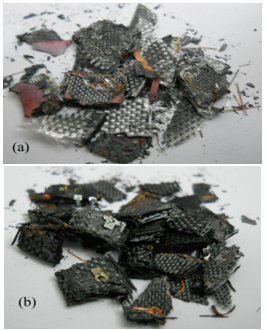 | Figure 8. Photographs of PCBs scrap (a)Burned (b)Pyrolysed at 600°C for 50mins |
3.5. Recovery of Copper by Leaching
- The comparison of copper recovery obtained from untreated, pyrolysed and air-burned samples by nitric acid leaching is presented (Figure. 9).
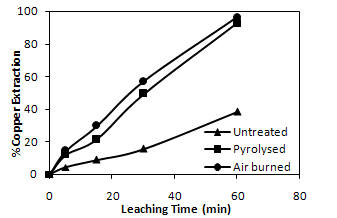 | Figure 9. Effect of thermal pre-treatment on metal recovery by leaching with time |
4. Conclusions
- The characteristics of low temperature pyrolysis of printed circuit boards subjected to air and nitrogen atmosphere was studied. The impact of the thermal pre-treatment on the subsequent hydrometallurgical recovery of copper from PCB by leaching was also examined. On the basis of the present study following conclusions can be drawn:1) The thermal treatment at the temperature of 300℃ does not have any marked influence on the release of plastics from PCBs. With increase in temperatures the amount of plastics removed increases. Temperature of about 500℃ and duration about 50 minutes are sufficient for the effective removal of volatile compounds of PCB.2) The pyrolysis behaviour can be divided into three stages. The first stage is up to temperature of 296℃ where no loss in the weight of the PCB scrap was observed. The next stage was between 296-500℃ where rapid decomposition of the PCB scrap took place. While the third stage was observed above 500℃ where the rate of decomposition became almost constant.3) The weight loss achieved under similar conditions by combustion was slightly higher than that of pyrolysis. Also the activation energy calculated indicated that combustion was more favourable than pyrolysis; however the difference was not large. 4) Copper recovery from the untreated sample was poor. However copper recovery obtained from combustion and pyrolysis were considerably higher indicating prior thermal treatment is necessary along with mechanical upgradation in order to improve the process efficiency, thus making pyrolysis as an economical alternative for recycling PCBs.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML