-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Metallurgical Engineering
p-ISSN: 2167-700X e-ISSN: 2167-7018
2012; 1(5): 88-95
doi: 10.5923/j.ijmee.20120105.04
Thermodynamics and Viscosity Aspects in Manganese Nodule Residue Smelting for Silicomanganese Production
N. S. Randhawa 1, R. K. Jana 1, N. N. Das 2
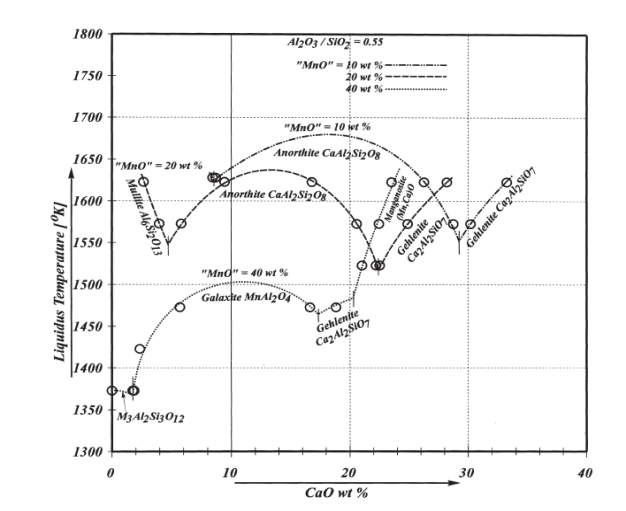
1Metal Extraction & Forming Division, CSIR-National Metallurgical Laboratory, Jamshedpur , 831 007, Jharkhand, India
2PG Department of Chemistry, North Orissa University, Baripada, 757 003, Orissa, India
Correspondence to: R. K. Jana , Metal Extraction & Forming Division, CSIR-National Metallurgical Laboratory, Jamshedpur , 831 007, Jharkhand, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Among several under trial processes for extraction of base metals (Cu, Co and Ni) from manganese nodules (MN), the reduction-roast ammoniacal leaching {NH4OH/(NH4)2CO3} process is considered very attractive. After the leaching of base metals from roast-reduced MN, a substantial quantity of residue left behind (about 70 wt. %, by mass) as wastes for disposal or further use. Smelting studies showed that leached manganese nodules residue (WMNR) can be successfully utilized as raw material for silicomanganese production after maintaining proper metallurgical aspects like thermodynamics, viscosity etc. Literature survey on slag liquidus and viscosity aspects of silicomanganese smelting has been done. The slag liquidus has been found to be closely associated to slag basicity i.e. (CaO+MgO/SiO2) showing significant increase above 0.7 slag basicity. Charge mix basicity of 0.2 was found to give maximum Mn and Si recoveries. Increasing or decreasing charge basicty from 0.2 resulted in lower metallic yield and Mn and Si recoveries. This has been explained with liquidus calculations of final slags obtained at those charge mix basicities. Addition of CaF2 during holding time i.e. time given after complete melting of charge mix was found to be beneficial. The optimum amount of CaF2 was 4% of WMNR+Mn ore blend, which gave rise to maximum Mn and Si recoveries and metallic yield. Further addition of CaF2 (>4%) produced a high basicity slag (basicity =0.83) having very high liquidus leading to decrease in metallic yield and Mn and Si recoveries.
Keywords: Leached Manganese Nodules Residue, Basicity, Smelting, Silicomanganese, Slag Liquidus, Viscosity
Cite this paper: N. S. Randhawa , R. K. Jana , N. N. Das , "Thermodynamics and Viscosity Aspects in Manganese Nodule Residue Smelting for Silicomanganese Production", International Journal of Metallurgical Engineering, Vol. 1 No. 5, 2012, pp. 88-95. doi: 10.5923/j.ijmee.20120105.04.
Article Outline
1. Introduction
- Wastes from mining and metallurgical industries are notorious for their hazardous impact on living beings. Hence, issues like processing of mining and metallurgical byproducts/wastes such as fines, lean grade ores, slags, dusts, sludges etc., their safe disposal and recovery of metallic values are considered critical from environmental, economic and social point of view[1]. The present work deal with the manganese nodules processing waste, generated after selective recovery of copper, nickel and cobalt by ammoniacal leaching of manganese nodules[2]. The leached residue of manganese nodules is a fine powdery oxide material. Several studies have been carried out to develop the technologies for complete utilization of waste manganese containing materials like high phosphorous manganese slag, high-carbon ferromanganese-byproduct metal etc[3-5]. However, use of such complicated materials require detailed thermodynamic analysis and their effect on partitioning of the main elements viz. Mn, Si etc., between the silicomanganese and slag in the industrial furnaces. The thermodynamic equilibrium conditions between alloy and slag determine the yield and grade of alloy in silicomanganese smelting.The MnO, SiO2, CaO, MgO and Al2O3 are the prevailing oxides taking part in silicomanganese smelting reactions in slag. The MnO and SiO2 undergo reduction by ‘carbon’ either as graphite or in SiC[6], which can be represented by Eqn (1) and Eqn. (2).
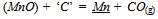 | (1) |
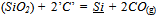 | (2) |
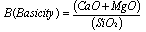 | (3) |
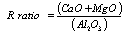 | (4) |
2. Thermodynamics and Viscosity Aspects in Silicomanganese Smelting
- As mentioned above, that reduction of MnO and SiO2 mainly takes place from slag; and therefore maintaining a sufficiently liquid slag with minimum viscosity at operating temperature i.e. at 1600 ±50℃ is a prerequisite for charge mix preparation. Additionally, the raw materials must be blended in such proportion that would produce slag with minimum MnO content and allow desired distribution of Mn and Si between alloy and slag phase. In silicomanganese production, smelting is carried out in acidic slag i.e. slag basicity<1. Several studies have been done to study the effect of slag basicity on Mn and Si distribution or Distribution Ratio i.e.[ratio of Wt. % metal in slag to that in alloy]. Equilibrium studies on distribution of Mn between Mn-Si-Fe-C alloys and MnO-CaO-MgO-SiO2-Al2O3 slag at 1500℃ under CO atmosphere revealed that an increase in basicity ratio of slag decreases the Mn distribution ratio[12]. In contrast, increase in silica concentration of the slag increases the Mn distribution ratio. The optimum slag basicity B, reported by Chaichenko et al[13] is in the range of 0.65-0.90. Addition of alumina to slag favours transfer of Mn to alloy leading to a low MnO slag. In the studies by Cengizler et al[14] activity data for Fe-Mn slags were modelled by applying neural nets at 1500℃ for slag compositions lying in the range of MnO 5–40%, CaO 4–35%, MgO 0.3–38%, SiO2 25–60% and Al2O3 2.5–7%. Their study concluded that activity coefficient of MnO in liquid slag region varies on both sides of unity. Studies by Tang and Olsen[15] and Ding and Olsen[16] also reported that addition of alumina to acidic slag decreases the equilibrium MnO content in the slag. In acid slags the ϒMnO < 1, whereas in basic slags the ϒMnO > 1, where ϒ is activity coefficient. The basicity of slag keeps on increasing up to the equilibrium value due to reduction of SiO2 to Si. On the other hand, R ratio practically remains constant throughout the smelting and should be more influencing to slag-metal equilibrium. Nikolaev[10] reported the optimum R value to be 2.5 whereas Emlin et al[17] concluded optimum R value in the range of 1.2-2.2. Eissa et al[5] studied the smelting of high Mn slag and observed the highest metallic yield and recoveries of manganese and silicon with initial R value of 1.8 by using dolomite as fluxing material. They also concluded that slag basicity adjusted by addition of dolomite gave higher metallic yield, manganese and silicon recoveries comparing with that obtained by adding either limestone or dolomite and limestone together. Equilibrium studies done by Ding and Olsen[16] concluded that the R ratio strongly influences the SiO2 activity of the slag and thereby the Si content of the alloy. Iso-activity lines of SiO2 in the system SiO2-CaO-Al2O3 are shown in Fig 1[18]. The lines representing constant silica activities in the slag are at the same time lines of constant silicon activity in equilibrated metal alloy. From the top apex of Fig 1 are drawn straight lines having constant CaO/Al2O3 ratios called R ratios. Thus R ratio is found to be much more important in SiMn production (Eqn. 4) than the lime basicity ratio (Eqn. 3).
 | Figure 1. Calculated (FACTSage) iso-activity lines of SiO2 in SiO2–CaO –Al2O3 slags at 1600℃ intersected by lines having constant R ratios (R=CaO/Al2O3)[18] |
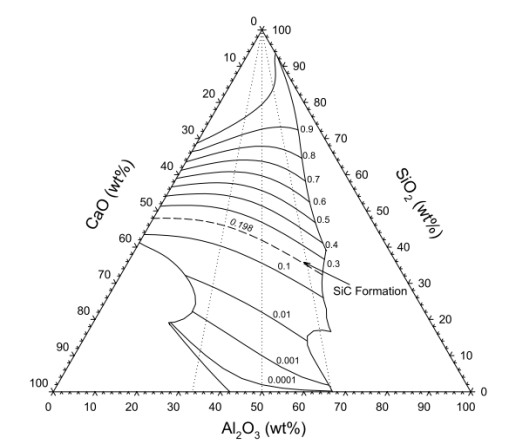
 | Figure 2. Complete equilibrium relations for ternary MnO-SiO2-CaO slags in equilibrium with Mn-Si-Csat alloys at PCO=1atm[18] |
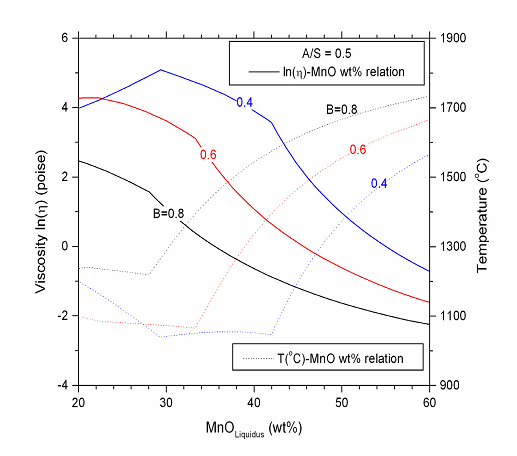 | Figure 4. Calculated viscosities along the liquidus with fixed A/S ratio and basicity (solid lines). The dotted lines represent the corresponding temperature in the solid lines[24] |
3. Leached Manganese Nodule Residue Smelting
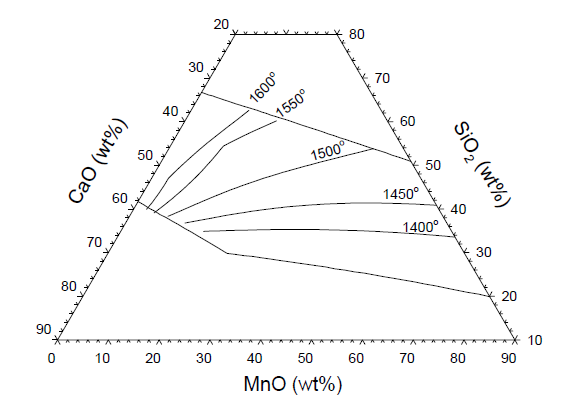
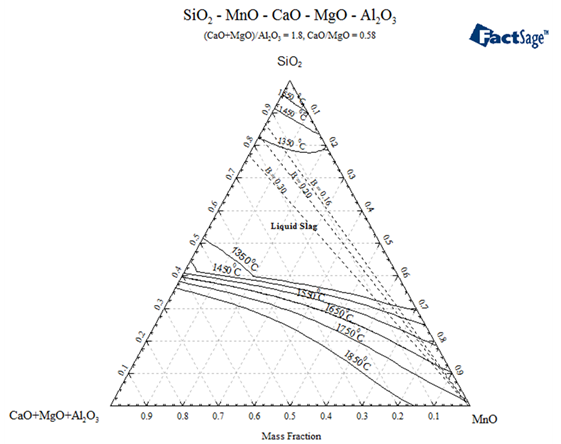 | Figure 5. Calculated (FACTSage) Slag-liquid projection at different temperatures for pseudo-ternary slag system (CaO+MgO+Al2O3)-SiO2- MnO, also showing the basicity lines for 0.16, 0.2 and 0.3 |
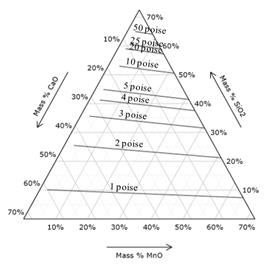 | Figure 6. Iso-viscosity line for (CaO+MgO)-(SiO2+Al2O3)-MnO slag system with 12.5 mass % Al2O3 and 15 mass % MgO using Urbains model[22] |
3.1. Role of Charge Basicity in Leached Manganese Nodule Residue Smelting
- An increase in the basicity of the slag increases the aMnO (basic oxide) decreases the aSiO2 and hence, more MnO and less SiO2 are reduced with increasing basicity of slag, which is also evident from aMnO and aSiO2 of initial slag calculated using Factsage 6.1, shown in Fig. 7. Thus, it is apparent that increasing basicity decreases equilibrium MnO and increases SiO2 in slag[8]. Therefore, optimum charge basicity is of utmost importance while smelting the MnO and SiO2 together.
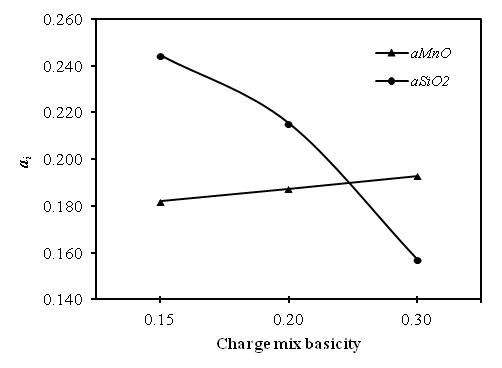 | Figure 7. Calculated aMnO and aSiO2 in the intial slags using Factsage |
| |||||||||||||||||||||||||||||||||||||||||
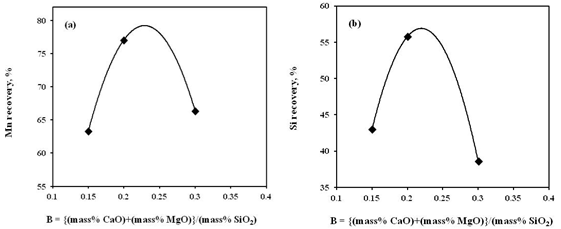 | Figure 8. Effect of charge mix basicity on recovery of (a) Mn and (b) Si in alloy |
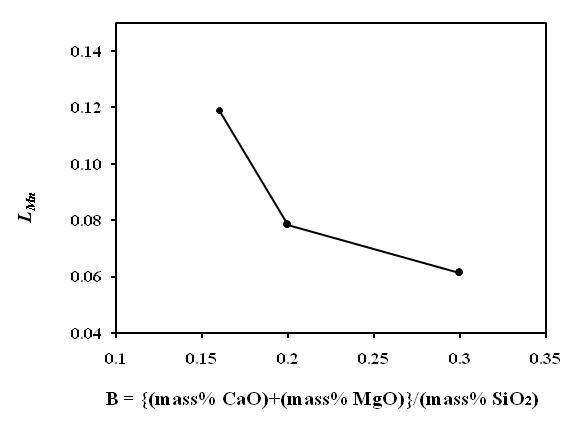 | Figure 9. Effect of charge mix basicity on Mn distribution between slag and alloy |
3.2. Effect of CaF2 Addition
- The chemical composition of alloys obtained from CaF2 addition smelting trails are given in Table 3. The acidic slag in silicomanganese smelting often poses difficulties in slag-alloy separation leading to lower yield and recovery. Therefore, CaF2 is often added in the acidic slag to decrease the viscosity. The CaF2 breaks the SiO2 network in the slag and improves slag fluidity. On the other hand, dissolution of CaF2 in the slag give rise to more CaO, which increases slag basicity as well as slag liquidus. Therefore, optimum amount of CaF2 must be ascertained before proceeding to commercial smelting trials. The CaF2 addition made in molten charge at initial charge mix basicity of 0.2, during holding time, found to be beneficial for smelting yield and recoveries. The Mn recovery (Fig. 10(a)) increased with addition of CaF2 up to 4% of WMNR:MO blend but resulted in decreasing Mn recovery with 6% CaF2 addition. The Si recovery (Fig. 10(b)) has also been found to increase with addition of 2% CaF2 and thereafter decreased with further addition of CaF2. The decreasing manganese distribution ratio (LMn) has been obtained up to 4% of CaF2 addition (Fig. 11), which depicts the proper slag metal separation. Addition of 6% CaF2 showed increased manganese distribution ratio (LMn).
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||
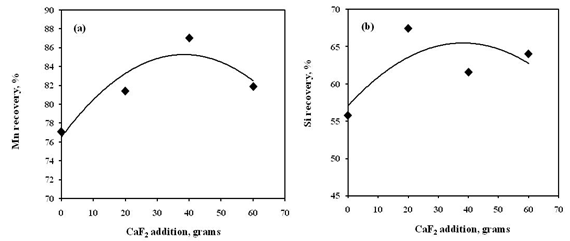 | Figure 10. Effect of CaF2 addition on recovery of (a) Mn and (b) Si in alloy |
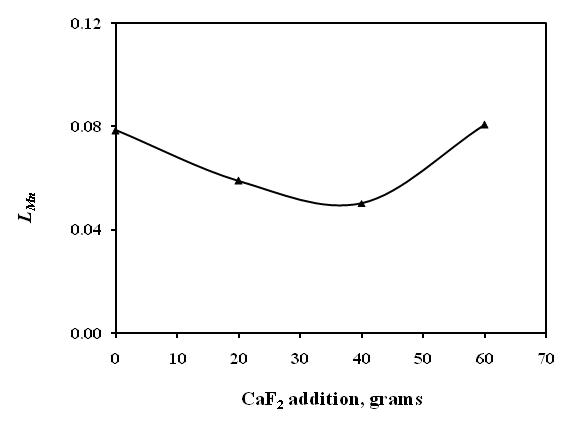 | Figure 11. Effect of CaF2 addition on Mn distribution between slag and alloy |
4. Conclusions
- Smelting studies have shown that leached manganese nodule residue (WMNR) is a potential raw material for producing standard grade silicomanganese alloy (Si16Mn63). The thermodynamics and viscosity aspects ofsilicomanganese smelting have been evaluated and applied to WMNR smelting. The higher metallic yield and Mn recoveries at charge mix basicity of 0.2 were satisfactorily correlated to slag liquidus calculated using software Factsgae. Addition of CaF2 up to 4% of WMNR+Mn ore blend during holding time was found to be beneficial to metallic yield and Mn recovery. Further CaF2 addition increased the slag liquidus resulting in lower metallic yield and Mn and Si recoveries. Thus thermodynamic and viscosity studies of WMNR smelting has been found to be useful for charge mix optimisation and maximisation of metal recovery in the production silicomanganese from manganese nodules leached residue.
ACKNOWLEDGEMENTS
- The authors thankfully acknowledge the permission from Director, CSIR-NML, Jamshedpur to publish this paper.
References
| [1] | Richard P.L. 1978, “Hazardous Solid Waste from Metallurgical Industries”. Environmental Health Perspectives, 27, 251-260. |
| [2] | Jana R.K., Pandey B.D. and Premchand 1999, “Ammoniacal leaching of roast reduced deep-sea manganese nodules”. Hydrometallurgy, 53, 45-56. |
| [3] | Voronov V.A. and Gavrilov V.A. 1996, “Efficiency of technology for smelting the silicomanganese duringutilization of secondary resources”. Publ: Russia, STAL' 1, 33-34. |
| [4] | Zhang H.T., Liu Y.J., Zhao D.X., Wang S.M., Wang S.Z., Xi J.S. and Wu K.H. 1995, “Silicon-manganese smelting using quarzite-coke as a reductant”. IRON STEEL (CHINA) 30(1), 63-66. |
| [5] | Eissa M., Fathy A., Ahmed A., El-Mohammady A. and El-Fawakhary K. 2004, “Factors affecting silicomanganese production using manganese rich slag in the charge”. In Proc. Conference of Tenth International Ferroalloys Congress, INFACON X (The South African Institute of Mining and Metallurgy: South Africa), pp 245-253. |
| [6] | Olsen S.E., Tangstad M. and Lingstad T. 2007, “Production of Manganese ferroalloys”. Tapir Academic Press: Trondheim, Norway. |
| [7] | Swinbourne D. R., Rankin W. J. and Eric R.H. 1995, “The effect of alumina in slag on manganese and silicon distribution in silicomanganese smelting”. Metall Mater Trans B, 26B, 59-65. |
| [8] | Turkdogan E.T. 1983. “Physico-chemical properties of molten slags and glasses”, The Metal Society, London. |
| [9] | Kor G.J.W. 1979, “Equillibria between liquid Mn-Si alloys and MnO-SiO2-CaO-MgO slags”. Metall. Mater. Trans. B, 10B, 367-364. |
| [10] | Nikolaev V.I. 1974, “Selection of optimum slag basicity in ferromanganese production”. Steel in USSR, 4, 299-302. |
| [11] | Tang K. and Tangstad M. 2007, “Modelling viscosities of ferromanganese slags”. in Proceedings Conference of Tenth International Ferroalloys Congress (INFACON XI), New Delhi, 344-357. |
| [12] | Cengizler H. 2003, “The manganese distribution between the metal and slag at 1500℃ in ferromanganese and silicomanganese production. Ore Dressing/Cevher Hazirlama, 9, 1-5. |
| [13] | Chaichenko A.A., Kashakashvili G.V., Arabuli I.A., Akhabadze T.V., Tskitashvili Yu.A., Veretennikov O.K., Murakhovskii V.V. and Sedov A.A, 1986, “Smelting of silicomanganese”. Otkrytiya,Izobret, 44, 82. |
| [14] | Cengizler H., Eric R.H. and Reuter M.A 1997, “Modelling of the activities and activity coefficients of Mn in ferromanganese slags”. In Proc. 5th International Conference on Molten Slags, Fluxes and Salts’97, Australia, Iron and Steel Society, Warrendale, PA, pp. 75–90. |
| [15] | Tang K. and Olsen S.E. 2006, “Computer simulation of equilibrium relations in manganese ferroalloy production”. Metall Mater Trans B, 37B, 599-606. |
| [16] | Ding W. and Olsen S.E. 2000, “Manganese and silicon distribution between slag and metal in silicomanganese production”. ISIJ Int., 40(9), 850-856. |
| [17] | Emlin B.I., Pogrebnyak A.I., Vodin I.I., Matyashenko N.K., Belan V.D., Sarankin V.A., Chupakhin Yu.M., Shaposhnik I.I. and Shchedrovitskii V.Ya. 1986, “Silicomanganese smelting”, Otkrytiya Izobret, 30, 86-88. |
| [18] | Olsen S.E. and Tangstad M. 2004, “Silicomanganese production–Process understanding” In Proc. Tenth International Ferroalloy Congress (INFACON X), Southern African Institute of Mining and Metallurgy, pp. 231-238. |
| [19] | Roghani G., Jak E. and Hayes P. 2003, “Phase-equilibrium data and liquidus for the system “MnO”-CaO-Al2O3-SiO2 at Al2O3/SiO2 of 0.55 and 0.65”. Metall Mater Trans B, 34B, 173-182. |
| [20] | Kubaschewsky O., Evans E.U. and Alcock C.B. 1979, “Metallurgical Thermochemistry”. 5th ed., Pergamon Press, Oxford. |
| [21] | Barin I. 1989, “Thermochemical data of pure substances”. VCH Verlagsgesellschaft mbH, D-6940 Weinheim, Part 2. |
| [22] | Seetharaman S., Mukai K. and Sichen Du 2004, “Viscosities of slags-An overview”. In Proc. VII International Conference on Molten Slags Fluxes and Salts, The South African Institute of Mining and Metallurgy, pp. 31-42. |
| [23] | Muller J. and Erwee M. 2011, “Blast furnace control using slag viscosities and liquidus temperature with phase equilibria calculations”. In Proc. Southern African Pyrometallurgy 2011, ed. R.T. Jones & P. den Hoed, Southern African Institute of Mining and Metallurgy, pp. 309-326. |
| [24] | Tang, K. And Olsen, S.E., 2007, “The effect of alumina in ferromanganese slag”, in Proceedings Conference of Eleventh International Ferroalloys Congress (INFACON XI) (New Delhi, India), pp 335-343. |
| [25] | Randhawa, N.S., Jana R.K. and Das N.N. 2011, “Manganese nodules residue: potential raw material for FeSiMn production”, In Proc.15th Int. Conf. on Non-ferrous Metal, Kolkata, India, pp. Tech. 9/1-Tech.9/9 |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML