-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Metallurgical Engineering
p-ISSN: 2167-700X e-ISSN: 2167-7018
2012; 1(5): 78-82
doi: 10.5923/j.ijmee.20120105.02
A Comparative Study on the Precipitation of Hydrated Alumina from Different Sources
Barsha Dash , B. C. Tripathy , I. N. Bhattacharya , T. Subbaiah
CSIR-Institute of Minerals and Materials Technology, Bhubaneswar, India
Correspondence to: Barsha Dash , CSIR-Institute of Minerals and Materials Technology, Bhubaneswar, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Hydrated alumina from three different sources like sodium aluminate liquor, waste aluminium dross and synthetic salt like aluminium sulfate were studied to obtain various form of aluminium hydroxide. Boehmite (Al2O3.H2O), a form of hydrated alumina, has better performance in obtaining alumina, as the enthalpy of dehydration of boehmite is less as compared to gibbsite due to the less number of water molecules in the crystal lattice as compared to gibbsite. Precipitation of hydrated alumina was also studied by using synthetic aluminium sulfate solution with aqueous ammonia and alternating the sequence of reagent addition. Subsequently, the variation in the pH of precipitation and followed by variation of the ageing temperature produced a range of hydrated alumina with different phases and crystallinities. In another option hydrated alumina was precipitated from the sulfuric acid leach liquor of waste aluminium dross by varying the pH of the precipitation followed by temperature of ageing.
Keywords: Hydrated Alumina, Sodium Aluminate, Waste Aluminium Dross, Aluminium Sulfate, Activated Alumina
Cite this paper: Barsha Dash , B. C. Tripathy , I. N. Bhattacharya , T. Subbaiah , "A Comparative Study on the Precipitation of Hydrated Alumina from Different Sources", International Journal of Metallurgical Engineering, Vol. 1 No. 5, 2012, pp. 78-82. doi: 10.5923/j.ijmee.20120105.02.
Article Outline
1. Introduction
- The research in the field of alumina is very interesting and encouraging as plenty of raw materials are available, which have to be exploited for newer applications as well as recovering the metal at a competitive cost. The process for the precipitation of boehmite (Al2O3.H2O) from sodium aluminate liquor instead of regular gibbsite (Al2O3.3H2O) precipitation is one such innovative option where reduction in the energy consumption can be investigated. The precipitation of crystalline boehmite from supersaturated sodium aluminate liquor was investigated[1-8] to ascertain its applicability. As the properties of the hydrated alumina can be steered by changing the method of preparation, more work can be done in this area. Variation in the condition of precipitation such as pH, temperature, ageing conditions etc. produces aluminium hydroxides of various compositions, structures, morphologies, etc[9-16]. Many investigators have also tried to modify the Bayer's process as well as to utilize raw materials other than the bauxite ore. Utilization of secondary sources of alumina is a search for alternate source to be used in future. Some of the attempts are already made in these directions[17-20].The paper deals with the precipitation of boehmite. The preparation of activated alumina from waste aluminium dross, which can be considered as an alternate secondary source, is a first step in advancing towards “from waste material to value-added products.” Aluminium salts solutions are also having practical use of making different varieties of hydroxides and activated materials.
2. Experimental
2.1. Materials
- Gibbsite was obtained from M/s National Aluminium Company, Bhubaneswar, India. Boehmite seed was prepared hydrothermally from the supplied gibbsite at 195°C for 4h. Aluminium granules and sodium hydroxide (AR) obtained from Merck, India. The additives like tartaric acid, oxalic acid and EDTA (Rankem, India), succinic acid, salicylic acid, glutaric acid and citric acid (Acros Chemical, India), aspartic acid, xylose, glucose and glycerol (Merck, India) were used in the precipitation process. Aluminium sulfate heptahydrate and 25% ammonia solution, used as the starting material, were supplied by Merck, India. Aluminium dross is a waste material obtained from aluminium melting plants. The <850 μm (Tyler 20) size particles were taken for the study.
2.2. Method
- The supersaturated sodium aluminate liquor was prepared by dissolving required amounts of aluminium granules in sodium hydroxide solution. The liquor was prepared for different Al2O3/ Na2O (A/C) ratios. In general 150g/L Al2O3 solution with A/C ratios of 1.0 was prepared by taking 150 g/L of Na2O (added as NaOH). Precipitation experiments were carried out in a 300 mL capacity stainless steel reactor having a fitting lid. Stirring speed of 250 ± 25 rpm was maintained in each experiment. To the aluminate liquor (100 mL) with pre-adjusted A/C ratio 100g/L boehmite seed was added and stirred continuously at stipulated temperature. The time period mentioned 8hrs except where it was varied. The additives were added at the time of seed addition.Aluminium sulfate solution (0.2M) was neutralized with 5% aqueous ammonia by alternate addition of the reagents lik 1. Ammonia added to salt, 2.Salt added to ammonia. Three end point pH were selected for both the cases. Half of the samples were aged with a temperature of 150℃ for 4h. The samples are named as A, B and C for pH 5, 7 and 10 of ammonia added to salt systme and D, E and F for pH 5, 7 and 10 of salt added to ammonia system. The experiments on the dissolution of dross in H2SO4 were carried out in a flat-bottomed glass reactor, which was placed on a magnetic stirrer with hot plate and temperature was maintained at 90 ± 2℃. The leach liquor was analysed by conventional EDTA–ZnSO4 method to determine the amount of alumina extracted. Precipitation and ageing were carried out exactly like the aforementioned procedure except the sequence adopted here is ammonia added to salt not the other one. After filtration each of the mentioned samples were washed with distilled water and dried in a hot air oven at 80°C for 48 h.
3. Results and Discussion
3.1. Precipitation of boehmite
- Precpitation of hydrated aluminas from sodium aluminate liquor is strongly dependent on A/C ratio which refers to the degree of supersaturation as the main driving force for precipitation. Seed provides surface on which newer crystals grow. Therefore, more seed amount and lesser seed size, more is the yield as illustrated in Figure1&2. When the A/C ratios varied from 0.75 to 1.2 it was found that at A/C ratio≥1 and when temperature was ≤80℃ the precipitated boehmite was mixed with gibbsite phase because at that condition supersaturation was more and gibbsite precipitation is kinetically more favoured (Figure 3).
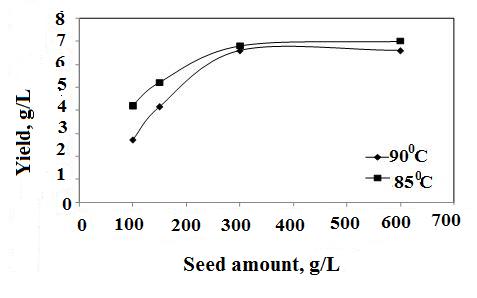 | Figure 1. Effect of seed quantity on boehmite yield, A/C = 1.0, 8h, 62.2μm (Dash et al., Hydrometallurgy, 2009) |
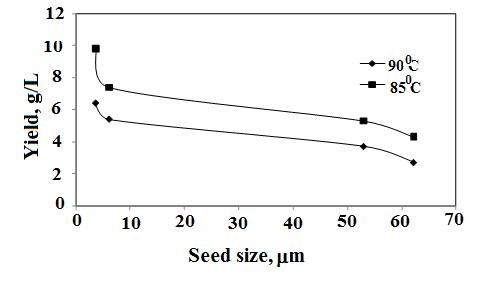 | Figure 2. Effect of seed size on boehmite yield, A/C= 1.0, 100 g/L, 8h (Dash et al., Hydrometallurgy, 2009) |
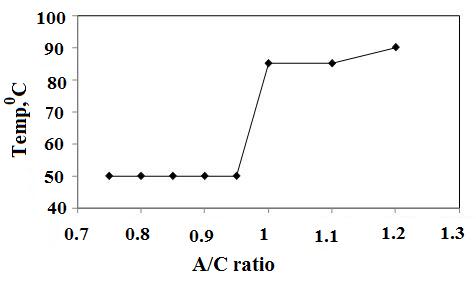 | Figure 3. Lowest temperature of boehmite (only) precipitation vs A/C ratio, 8 h, 100 g/L, 62.2 μm (Dash et al., Hydrometallurgy, 2009) View Within Article |
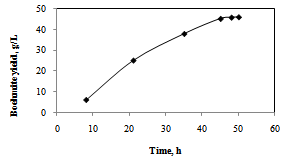 | Figure 4. Effect of precipitation time on boehmite yield, A/C = 1.1, temp.=80℃, tartaric acid=6g/L |
3.2. Precipitation of Hydrated Alumina from Sulfate Salt of Aluminium
- X-ray diffraction patterns of A0 and B0 (without ageing) were found to be broad indicating an amorphous structure and having gel like characteristics. Thus no crystalinity was observed in these cases. However, with higher ageing temperature like 150℃, the XRD-patterns of A150 and B150 showed sharp peaks indicating a crystalline structure as shown in Figure 5. Thus crystalinity was found to develop on ageing at higher temperature. The diffraction patterns of the materials obtained at pH 10 (C0 and C150) were found to be distinctly crystalline bayerite for both aged and unaged samples.The conditions are different in the case when salt added to ammonia. The XRD pattern of all the samples (D, E and F) was found to be crystalline as shown in Fig.6. Even the freshly precipitated samples (D0, E0 and F0) i.e. the unaged ones were also crystalline. The peaks are however broad enough showing low maturity in the crystal growth. When the materials were aged at 150℃ proper crystallinity was marked in the X-ray diffraction pattern. It was observed that in the system where ammonia is added to salt the crystallinity is either a function of pH or ageing. But if the addition is reversed precipitate crystallinity was obtained.
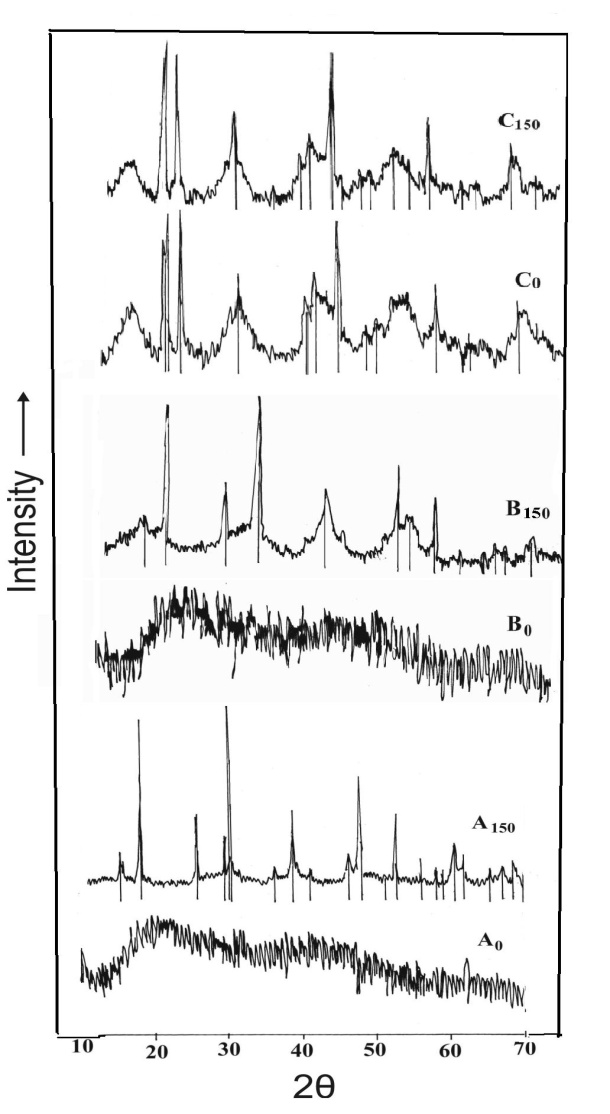 | Figure 5. XRD pattern of samples of ammonia added to salt without ageing (subscript 0) and with ageing at 150℃ (subscript 150) |
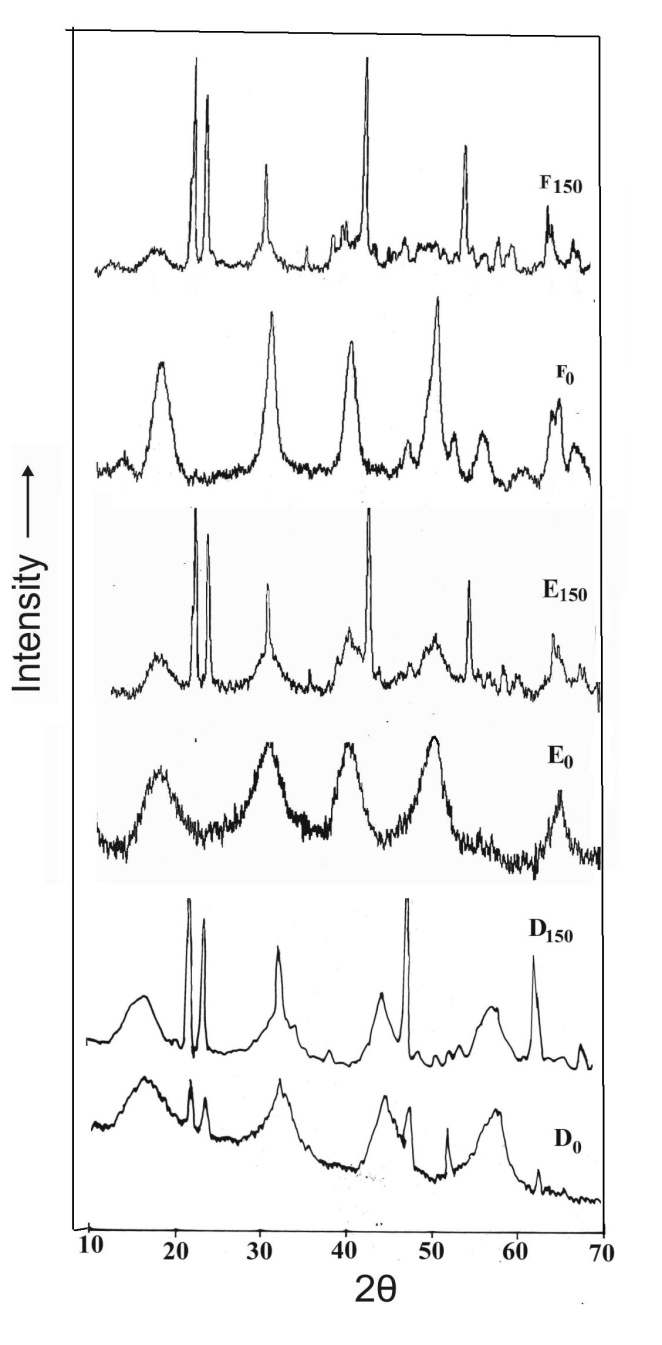 | Figure 6. XRD pattern of samples of salt added to ammonia without ageing (Subscript 0) and with ageing at 150℃ (subscript 150) |
3.3. Precipitation of Hydrated Alumina from Waste Aluminium Dross
- The typical dross sample taken was found to be containing 64.8% of alumina. It was found to be containing both alumina and Al metallic phases. The leaching with sulfuric acid brings the aluminium values in any form to ionic form in the leach liquor. The following reactions are supposed to be taking place-
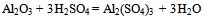 | (1) |
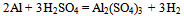 | (2) |
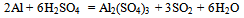 | (3) |
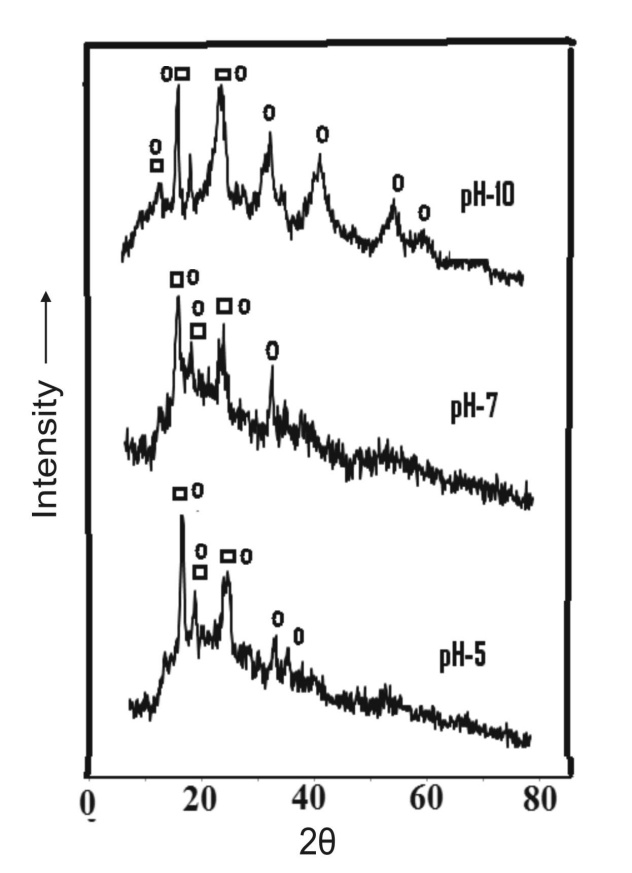 | Figure 7. XRD pattern of the precipitated aluminium hydroxide without ageing; : aluminium hydroxide, : iron hydroxide |
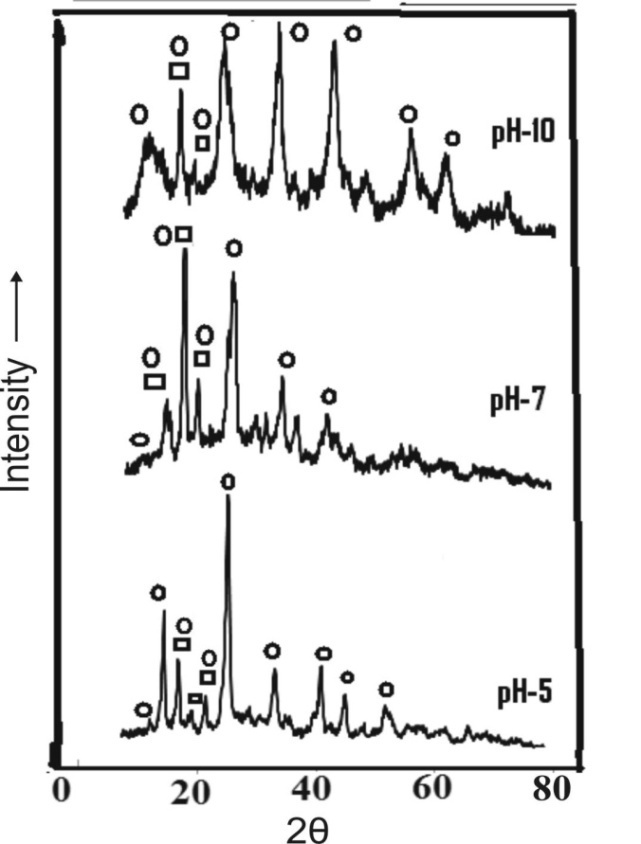 | Figure 8. XRD pattern of the precipitated and aged aluminium hydroxide  ;aluminium hydroxide, ;aluminium hydroxide,  : iron hydroxide : iron hydroxide |
|
References
| [1] | K. Wafer, C. Misra, “Oxides and hydroxides of aluminium”, Alcoa Tech. Paper No. 19, Rev., 1987, Pittsburg. |
| [2] | E.A. Elkatatatny, S.A. Halawy, M.A. Mohamed, M.I. Zaki, “A novel synthesis of high-area alumina via H2O2 - precipitated boehmite from sodium aluminate solutions”, Wiley publications, J Chem Technol Biotechnol, vol. 72, pp. 320–328, 1998. |
| [3] | D. Panias, I. Paspaliaris, A. Amanatidis, H.W. Schmidt, A.l. Hollnagel, “Boehmite process: An alternative technology in alumina production”, Wiley Publications, Light Metals, pp. 97–103, 2001. |
| [4] | D. Panias, P. Asimidis, I. Paspaliaris, “Solubility of boehmite in concentrated sodium hydroxide solutions: model development and assessment”, Elsevier B.V.,Hydrometallurgy, vol. 59, pp. 15-29, 2001. |
| [5] | C. Skoufadis, D. Panias, I. Paspaliaris, “Kinetics of boehmite precipitation from supersaturated sodium aluminate solutions”, Elsevier B.V., Hydrometallurgy, vol. 68, pp. 57-68, 2003. |
| [6] | B. Dash, B. C. Tripathy, I. N. Bhattacharya, S. C. Das, C. R. Mishra, B. S. Pani, “Effect of temperature and alumina/ caustic ratio on precipitation of boehmite in synthetic sodium aluminate liquor”, Elsevier B.V., Hydrometallurgy, vol. 88, pp. 121-126, 2007. |
| [7] | B. Dash, B.C.Tripathy, I.N.Bhattacharya, S.C.Das, C.R. Mishra, B.K.Mishra, “Precipitation of boehmite in sodium aluminate liquor”, Elsevier B.V., Hydrometallurgy, vol. 95, pp. 297-301, 2009. |
| [8] | B. Dash, B.C.Tripathy, I.N.Bhattacharya, B.K.Mishra, “Additive action on boehmite precipitation in sodium aluminate liquor”, Royal Society of Chemistry, Dalton Trans, vol. 39, pp. 9108-911, 12010. |
| [9] | J. K. Pradhan, I. N. Bhattacharya, S. C. Das, R. P. Das, R. K. Panda, “Characterisation of fine polycrystals of metastable η-alumina obtained through a wet chemical precursor synthesis”, Elsevier B.V., Mater Sci Eng B, vol. 77, pp. 185-192, 2000. |
| [10] | J. K. Pradhan, P. K. Gochhayat, I. N. Bhattacharya, S.C.Das, “Study on the various factros affecting the quality of precipitated non-metallurgical alumina trihydrate particles”, Elsevier B.V., Hydrometallurgy, vol. 60, pp. 143-153, 2001. |
| [11] | I. N. Bhattacharya, J. K. Pradhan, P. K. Gochhayat, S. C. Das, “Factros controlling precipitation of finer size alumina trihydrate”, Elsevier B.V., Int J Mineral Processing, vol. 67, pp. 109-124, 2002. |
| [12] | K Okada. T. Nagashima, Y. Kameshima, A. Yasumori, T.Tsukada, “Relationship between Formation Conditions, Properties, and Crystallite Size of Boehmite”, Elsevier B.V., J Colloid Interface Sci, vol. 253, pp. 308-314, 2002. |
| [13] | S. Musić, Đ. Dragčević, S .Popović, “Formation of boehmite via precipitation from aqueous solutions”, Elsevier B.V., Mater Lett, vol. 24, pp. 59-64, 1995. |
| [14] | S. Musić, Đ. Dragčević, S. Popović, N. Vdović, “Microstructural properties of boehmite formed under hydrothermal conditions”, Elsevier B.V., Mater Sci Eng B, vol. 52, pp. 145-153, 1998. |
| [15] | D.A. Georgantas, H.P. Grigoropoulou, “Orthophosphate and metaphosphate ion removal from aqueous solution using alum and aluminum hydroxide”, Elsevier B.V., J Colloid Interf Sci, vol. 315, pp. 70-79, 2007. |
| [16] | A. Kocjan, K. Krnel, T .Kosmač, “The influence of temperature and time on the AlN powder hydrolysis reaction products”, Elsevier B.V., J European Ceram Soc, vol. 28, pp. 1003-1008, 2008. |
| [17] | L.W.Garret, U.S. Patent- 4,337,228, Appl. No. 06/271558, June 1982. |
| [18] | J.A. Huckabay, U.S. Patent-4,434,142, Appl. No. 06/426200, February 1984. |
| [19] | B.R.Das, B.Dash, B.C.Tripathy, I.N.Bhattacharya, S.C.Das, “Production of η-alumina from waste aluminium dross”. Elsevier B.V., Miner Eng., 20, pp. 252-258, 2007. |
| [20] | B.Dash, B.R.Das, B.C.Tripathy, I.N.Bhattacharya, S.C.Das, “Acid dissolution of alumina from waste aluminium dross” Elsevier B.V., Hydrometallurgy, vol. 92, pp. 48-53, 2008. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML