Abhilash 1, S. C. Pal 2, K. D. Mehta 1, B. D. Pandey 1, T. R. Mankhand 2
1CSIR-National Metallurgical Laboratory, Jamshedpur, 831007, India
2Department of Metallurgical Engineering, Indian Institute of Technology (Banaras Hindu University), Varanasi, 221005, India
Correspondence to: Abhilash , CSIR-National Metallurgical Laboratory, Jamshedpur, 831007, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Bioprocessing for copper recovery from a lean grade chalcopyrite ore of Malanjkhand Copper Project (MCP) is reported. This ore with 0.3-0.4% copper, which is adjacent to the rich grade ore (09-1.0% Cu), available in large amount is untouched for want of a suitable option, can be utilized to meet the growing demand of the metal in the country. Different leaching parameters such as pH, temperature, pulp density, particle size, etc; were optimised in shake flask experiments using Acidithiobacillus ferrooxidans (A.ferrooxidans) isolated from the source mine water. Sterile sets were also run to assess the contribution of chemical leaching in parallel with bioleach sets. The leaching under sterile control conditions recorded poor metal recoveries. In bioleach experiments, leaching of copper showed increasing trend with dissolved ferric iron in solution indicating the role of indirect leaching mechanism also. A high redox potential (674mV) observed during the bioleaching may be attributed to the enhanced bacterial oxidation thereby resulting in 72% Cu bio-recovery in 35 days at 35℃, pH 2, 5%PD and with <50µm ore particles. With the coarser size of the ore, the copper recovery was comparatively lower under the similar conditions. XRD phase identification of the ore and the leach residue showed the formation of jarosite limiting the bio-dissolution of the metal.
Keywords:
Chalcopyrite, Bio-leaching, Acidithiobacillus ferrooxidans, Ferric ions, Jarosite
Cite this paper:
Abhilash , S. C. Pal , K. D. Mehta , B. D. Pandey , T. R. Mankhand , "Bioprocessing of a Low-grade Chalcopyrite Ore by the Isolate of Acidithiobacillus Ferrooxidans", International Journal of Metallurgical Engineering, Vol. 1 No. 5, 2012, pp. 72-77. doi: 10.5923/j.ijmee.20120105.01.
1. Introduction
Copper ore of Malanjkhand copper mine is basically of granitic origin with thin vein-lets of quartz (rock type) and has a complex mineralogy. The rich grade ore (0.9 – 1.0% Cu) being mined is concentrated by flotation to produce chalcopyrite concentrate which is the main feed-stock for Hindustan Copper Limited (HCL) plants in the country. Adjacent to the main ore-body, the lean grade material (~2.5-3.0 millionT) containing 0.3-0.4% Cu is estimated which remains untapped because it is not economically attractive by the conventional flotation-smelting technology. Bio-leaching processes may be considered as possible alternative to treat this low-grade and complex ore which is often considered most suitable for such materials. Earlier, attempts were made[1,2] to treat MCP ores, particularly the overburden material which were essentially a mixed copper oxide-sulphide (chalcopyrite) and also the lean grade ore. Although, the copper bio-recovery from the mixed ore[1] was observed to be 50-60% in 60 days on bench scale and 40-50% in column, the same could not be established in large column and heap leaching experiments conducted at the dump site of MCP. In fact, copper dissolution was found to be 19% on tonnage scale by Agate[1], whereas recovery was 16-17% only in 9 months time in large heaps of 2000T at MCP. The bio-leaching of lean grade MCP ore of quartzitic origin has also been investigated to a limited extent with 80% recovery in 40 days in shake flasks[2]. In the bio-leaching process, the proper contact of ore with the leach liquor is essential for fluxes of reactants and products, such as bacteria, dissolved gases (O2 and CO2), solubilized metals and sulphur species. Ferric iron is an important oxidizing agent for the bacterial leaching of sulphide minerals[3-5]. Soluble iron species are the main determinants of redox potential, with active iron-oxidizing bacteria (Acidithiobacillus ferrooxidans) contributing to high Fe3+/Fe2+ ratio. Precipitation of ferric iron in the leaching system may suppress the metal solubilizaton by preventing the contact between the leaching agent and the mineral[6-9]. The bacterial leaching process requires acidic conditions, the acidity often being produced by the oxidation of sulphides/pyrite and hydrolysis of ferric iron[10-12]. The acid may, however, be neutralized in various acid-consuming reactions, such as the leaching of silicate minerals.As such the bio-leaching of the lean MCP ore particularly those of granitic origin as the main source of chalcopyrite containing host requires serious efforts so as to arrive at the conditions suitable for exploitation. Therefore, the present study was taken up as a part of CSIR (New Delhi) network project by involving different organizations in India. In this direction, some aspects of R&D work being carried out at CSIR-National Metallurgical Laboratory using the native bacteria viz. Acidithiobacillus ferrooxidans, are presented in this paper. Microbiological, chemical and physical factors influencing the bio-leaching are included in the text. Details on optimisation of process parameters in the bio-leaching of copper are also presented.
2. Experimental
2.1. Materials
Lean grade copper ore, containing 0.32%Cu, collected in the form of lumps from Malanjkhand copper mine (Madhya Pradesh) were crushed, ground and passed through a sieve of 150µm size. The bio-leaching experiments were mostly carried out with the sieved material (<50µm) unless stated otherwise. A representative sample was then prepared by coning and quartering for chemical analysis by atomic absorption spectrometer. A known amount of ore was also passed through different screens to get the sieve analysis fractions, the chemical analysis of which is given in Table-1. The phase identification by XRD showed that CuFeS2, FeS2(pyrite) and silica were the major mineral phases and Cu5FeS4 (bornite) as a minor mineral phase in the ore.| Table 1. Chemical analysis of different sieve fractions of copper ore from Malanjkhand mine |
| | Particle size (µm) | Composition (%) | | Cu | Ni | Fe | | 150-76 | 0.321 | 0.228 | 3.90 | | 76-50 | 0.316 | 0.225 | 3.92 | | <50 | 0.320 | 0.230 | 3.91 |
|
|
2.2. Methods
The micro-organism used in this study was a wild strain of Acidithiobacillus ferrooxidans (A.ferrooxidans), isolated from the MCP mine water sample using FeSO4.7H2O as a substrate in 9K nutrient medium[7]. Analytical Reagent Grade chemicals such as FeSO4.7H2O, MgSO4.7H2O, Ca (NO3)2, KCl, K2HPO4, and NH4 (SO4) were used for making 9K media. Other chemicals used were also of Analytical Reagent Grade. The culture thus derived was sub-cultured and then used for inoculation in subsequent bioleaching experiments. Cell count of the bacteria was determined by Petroff-Hauser Counter in a biological microscope. Unless otherwise stated, the bench scale leaching experiments were carried out in Erlenmeyer flasks in incubator shaker at 35+2℃. The bioleaching tests were carried out in 500 ml flasks with 200 ml of total solution, the flasks were inoculated with 10 %(v/v) of enriched liquid culture containing 1×108 cells/ml. General conditions like 35℃temperature, pH 2 and pulp density (PD) 5 % (w/v) were maintained unless otherwise stated. The flasks were incubated at the desired temperature with shaking at 100 rpm under orbital motion. A 0.5 ml of the supernatant solution was taken for analysis of Cu, Ni and Fe by Atomic Absorption Spectrometer. The ferrous ion concentration in the solution was determined by titrating against standard potassium dichromate solution with barium diphenylamine sulfonate (BDAS) as indicator. Normal losses due to evaporation were periodically compensated by the addition of distilled water. All the inoculated sets had their corresponding sterile control sets prepared under the same conditions excepting bacteria. During experiments samples were mostly taken at 5 days intervals for chemical analysis. The pH of the leach solution was maintained on alternate days with 10N H2SO4. Upon termination of the leaching experiments, the solid residues were dried and samples were taken for chemical analysis and XRD phase identification. The solid residues were dissolved in HCl-HNO3 mixture and analyzed by AAS. Redox potential (Eh) during the leaching experiments was measured with a Pt electrode using saturated calomel electrode (SCE) as a reference.
3. Results and Discussion
In the present work the bench scale study was performed to optimize the critical parameters for bioleaching of copper from the lean grade copper ore of Malanjkhand Copper Project. The details are presented and discussed below. The growth of bacteria (A.ferrooxidans) isolated in 9Kmedia from the source mine water was monitored by estimating the oxidation of Fe+2 with time. Total iron was oxidized in 7 days as shown in Fig.1 indicating the exponential growth of Acidithiobacillus ferrooxidans. The cell count increased from 5x106 to 6x108cells/mL in 7days; the lag phase being noticed till day 2. In subsequent sub-culturing, the growth was completed in 3-4 days and the A.ferrooxidans was in the active state. This sub-cultured A.ferrooxidans was used in the experiments for bio-leaching without adaptation to the ore because the strain was isolated from the copper mine water and was expected to be adapted to the prevalent environment – the ore body. Bacteria (A.ferrooxidans) was sub-cultured at 35℃ also to conduct bio-leaching experiments at this temperature.
3.1. Effect of pH
pH is one of the important parameters which affects the bioleaching process. Bioleaching of copper was done at different pH in the range 1.5-2.5 at a solid-liquid ratio of 1:20(w/v) and temperature of 25℃. Data plotted in Fig.2 showed that the extent and rate of recovery was maximum at pH 2.0, although copper bio-dissolution at pH 1.7 was quite near. Copper recoveries at pH 1.5 and 1.7 were mainly governed by increase in bacterial oxidation by direct mechanism involving attachment of A.ferrooxidans on the surface of sulphide mineral. At pH 2.0, the bio-action of A.ferrooxidans on the ore is reported to be maximum[10-12]. The copper recoveries of 40% and 37% were observed at pH 2.0 and 1.7 respectively in 27days. Thereafter, copper recoveries decreased with increase in pH. The lower recovery of copper at the higher pH of 2.5 may be attributed to the formation of jarosite on the ore particles, which was identified by XRD phase analysis.
3.1.1. Acid Consumption during Leaching
Acidithiobacillus ferrooxidans produces acid from sulphide minerals but some acid must be present initially for driving the leaching in forward direction. As the amount of sulphur in the copper ore is 2.83%, auto-generation of acid is expected to be quite lesser. Consumption of sulphuric acid (10N) during bioleaching of copper ore at 25oC is shown in Fig. 3. It is clear that initial acid requirement for leaching was sufficiently high which were 4.23 ml at pH 1.5 and 2.12 ml at pH 1.7. The acid requirement at pH 2.0 and above was moderately lower. Due to higher activity of bacteria at pH 2.0 more acid was produced and acid consumed was about 1.18 ml for 10 g of copper ore. The sterile control experiment carried out at pH 2.0 showed that 75% more acid was consumed in chemical leaching than bioleaching of copper ore.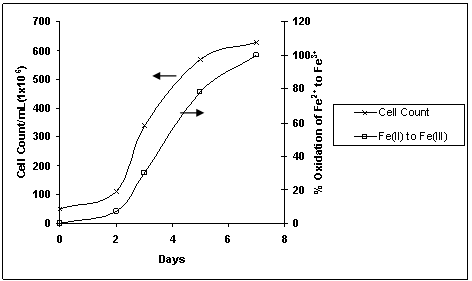 | Figure 1. Change in Cell count with iron oxidation rate for isolation of A.ferrooxidans from source mine water at pH 2.0 and 25℃ |
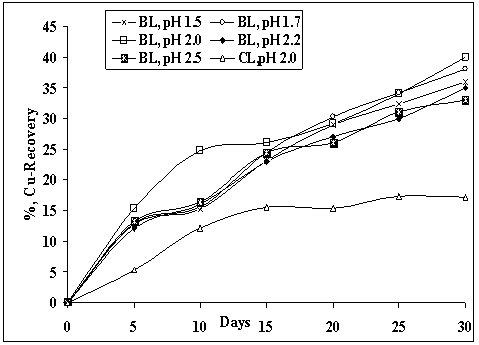 | Figure 2. Recovery of copper at different pH using A.ferrooxidans isolate from mine water at 25℃, 5%PD and particle size of <50µm |
The bioleaching of copper from chalcopyrite is reported[13-15] to involve both direct and indirect leaching mechanism. The direct mechanism proceeds through the attachment of A.ferrooxidans on the mineral surface to oxidize the metal. | (1) |
There is growing agreement that the biooxidation of sulphide minerals also involves oxidative ferric reaction with the mineral, which essentially represents indirect leaching mechanism as:  | (2) |
This apart from dissolution of the metal sulphide ions, produces ferrous iron and elemental sulphur (So). It is this ferrous iron and the elemental sulphur that form the substrate for microbial growth according to reaction:  | (3) |
And: | (4) |
The ferric iron thus formed is hydrolyzed in aqueous solution if pH is higher. | (5) |
 | (6) |
Reaction (3) increases the pH, but the reaction (5) and (6) reduce and stabilizes it. Therefore, the extent of ferric iron hydrolysis is dependent on pH.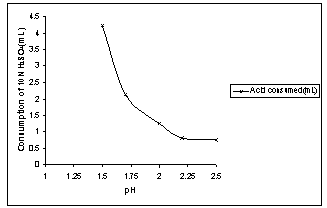 | Figure 3. Consumption of H2SO4 (10N) in bio-leaching at different pH in 30days, 5%PD and 25℃ |
3.2. Effect of Pulp Density (PD)
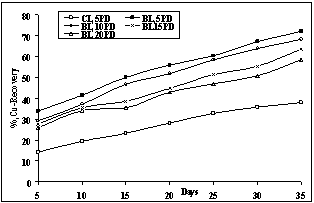 | Figure 4. Effect of Pulp Density on bioleaching of copper with A.ferrooxidans in 35 days at 35oC, pH 2 and <50µm size particles.[CL: Chemical Leaching, BL:Bio-leaching] |
Effect of pulp density in the range 5 - 20%(w/v) on the biodissolution of metal was investigated using 10% (v/v) isolate of A.ferrooxidans in 200 ml leaching medium with <50µm size particles at pH 2 and 35℃ while shaking at 100rpm. From the results shown in Fig.4, it is clear that the bio-recovery of copper is much higher at the lower pulp density of 5% (w/v) of the solution. The maximum copper bio-recovery was found to be 72 % with 5%PD in 35 days as compared to ≈ 40% Cu recovery in chemical leaching under this condition. At the pulp density of 10%, copper dissolution was 66% during the same period. Copper recovery decreased with increase in pulp density, which may be attributed to the lack of oxygen availability and increased concentration of metal ions which might have caused toxicity to bacterial growth at higher pulp densities. Thus, pulp density of 5%(w/v) was maintained in subsequent experiments.
3.3. Effect of Particle Size
Studies on effect of particle size on the recovery of metal are shown in Fig.5. It is apparent that increase in fineness to <50 µm increased the copper recovery to 72% which was lower at 67.6% with the particles of 76-50µm size. The still coarser size (150-76µm) showed copper bio-dissolution of ~41% in 35 days time which was near to the control (chemical) leaching with finest size (<50µm) ore particles. This could be mainly due to better permeation of the leachant to oxidize the copper sulphide (chalcopyrite) present in the ore with increased surface area. Finer particles were increasingly exposed to lixiviant that dissolved copper from the chalcopyrite phase. The concentration of ferrous ions which was oxidized by bacterial action (Fig.6) was much lower in case of leaching of finer size particles (<50µm) than that of coarser size (150-76µm) ore. Almost complete oxidation to ferric state in 5days for finest size material was responsible for high copper bio-recovery through chemical oxidation by indirect leaching mechanism[13,14]. In the sterile control experiment, Fe(II) concentration remained the same beyond 5days and therefore lower metal recovery was observed.
3.6. Effect of Temperature
In general, chemical reaction rate increases as the temperature increases, but the Acidithiobacillus ferrooxidans strains are mesophilic in nature and they survive or thrive in the temperatures range 20-35℃. Influence of varying temperature on bioleaching at 5% PD using 10% (v/v) isolate of A.ferrooxidans at pH 2, was studied. The maximum copper recovery was found to be 72 % at 35℃ in 35 days as shown in Fig.7. The copper recovery fell to 57% at 30℃ under this condition. During bioleaching, redox potential varied from 316 to 674 mV in 35 days, whereas in control leaching it increased from 325 to 534mV. High Eh in the bio-leaching is associated with strong oxidizing conditions and is therefore indicative of increased metal recovery. | Figure 5. Effect of particle size on bioleaching of copper with A.ferrooxidans at 35℃, pH 2 and 5% PD |
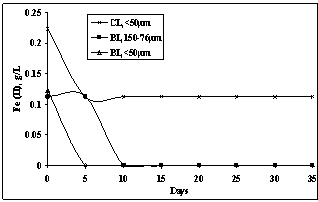 | Figure 6. Change in ferrous ion concentration with particle sizes at 5%PD, 2.0pH and 35℃ temperature |
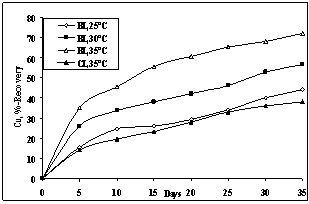 | Figure 7. Effect of temperature on bioleaching of copper at PD 5% w/v, pH 2 and <50µm size |
The XRD identification of the phases can be seen from Table 2. The leach residues obtained in the bio-leaching at 2.0pH contained chalcopyrite and pyrite as the minor phases along with bornite. Besides, the residue also contained hydronium jarosite[H3OFe3(SO4)2 (OH)6] in major amount which was formed during the leaching process. Jarosite precipitation can be indicated by equation (7): | (7) |
where X+ is a monovalent cation (generally K+, Na+, NH4+ or H3O+). Precipitation of jarosite was lower at pH 2, but at pH 2.5 increased precipitation of the same was observed. Higher jarosite formation at the surface of the ore at higher pH resulted in hindered action of lixiviant causing lower metal recovery as discussed earlier.| Table 2. XRD phase identification of copper ore before and after bioleaching at 35oC, 35 days, pH 2 and <50 μm particle size |
| | Sample | Phases Identified | | Copper ore | Major: chalcopyrite and pyrite (associated with copper ore), silica | | Minor: bornite | | Bioleach residue | Major: hydronium jarosite, silica | | Minor: chalcopyrite, pyrite |
|
|
4. Conclusions
On the basis of bench scales studies for bio-leaching of copper from lean grade MCP ore, following conclusions are drawn: ● Copper dissolution is significantly enhanced by the presence of wild strains of A.ferrooxidans isolated from the mine water, at pH 2 and 35℃ temperature. ● Low acid consumption at 2.0pH shows the increased formation of sulphuric acid due to enhanced bacterial activity of A.ferrooxidans.● The high redox potential in the range 316-674 mV in the presence of A.ferrooxidans indicates strong oxidizing conditions prevailing in the system at 35℃ and 2.0pH as compared to chemical leaching under the sterile conditions. High metal recovery is attained with the finer size (<50 μm) and higher temperature of 35℃.● The maximum copper bio-recovery achieved is 72% at pH2 and 35℃ with the ore particles of <50 μm size in 35 days when 5%(w/v) pulp density is maintained during leaching.● Bacterial leaching of chalcopyrite appears to follow both direct and indirect leaching mechanism, the role of latter is being clearly indicated by the presence of high concentration of ferric ions and faster oxidation rate of Fe(II).
ACKNOWLEDGMENTS
The authors are thankful to the Director, NML Jamshedpur for giving permission to publish the paper. Thanks are also due to the management of MCP, Hindustan Copper Limited for providing the ore and mine water samples.
References
| [1] | K.A.Natarajan, Biohydrometallurgy-Theory & Practice, 1994. |
| [2] | L. Patnaik, R.N. Kar and L.B. Sukla, Influence of pH on bioleaching of copper and zinc from complex sulphide concentrate using Thiobacillus ferrooxidans, Trans. Indian. Inst. Met., 54(4), 139-144, 2001. |
| [3] | G. Rossi, Biohydrometallurgy, Mc Graw-Hill, Hamburg, 1990. |
| [4] | A. Schippers and W.Sand, Bacterial Leaching of metal sulfides proceed by two indirect mechanisms via thiosulfate or via polysulfides and sulfur, Appl. Environ. Microbiol., 65,319-321, 1999. |
| [5] | M.P. Silverman, Mechanism of bacterial pyrite oxidation, J. Bacteriol., 94, 1046-1051, 1967. |
| [6] | K.A. Natarajan, Electrochemical aspects of bioleaching of base-metal sulphides, In Microbial Mineral Recovery, Eds. H.L.Ehrlich and C.L.Brierley, McGraw-Hill Pub. Co., New York, 79-106, 1990. |
| [7] | K.D.Mehta, B.D.Pandey and Premchand, Bio-assisted leaching of copper, nickel and cobalt from copper converter slag, Mat. Trans, JIM, 40 (3), 214-221, 1999. |
| [8] | A. Das, J.M. Modak and K.A. Natarajan, Studies on Multi-metal ion tolerance of Thiobacillus ferrooxidans, Min. Engg. 10(7), 743-749, 1997. |
| [9] | K.A. Natarajan, Biotechnological innovations in nonferrous extraction, In Proc. Nonferrous Extractive Metallurgy in the New Millennium, Eds. P.R.Rao, R.Kumar, S. Srikanth and N.G. Goswami, NML, Jamshedpur Press, 1-20, 1999. |
| [10] | Natarajan K.A. and Narayanmoorty, Bioleaching of enriched chalcopyrite tailing, Trans Ind inst.Met., 35(5), 353, 1980. |
| [11] | D. Pradhan, S. Pal, G. Roy Choudhury, T. Das, L. B. Sukla, Bioleaching of low grade copper ore using indigenous microorganism, Ind. J. Chem. Technol. 15, 588-592, 2008 |
| [12] | M.Mishra, S.Singh, T. Das, R. N. Kar, L.B.Sukla, B.K.Mishra, Bio dissolution of copper from Khetri lagoon material by adapted strain of Acidithiobacillus ferrooxidans, Korean J. Chem. Engg., 25,05-13, 2008. |
| [13] | G.S. Hansford and T.Vargas, Chemical and electrochemical basis of bioleaching processes, Hydrometallurgy, 59, 135-145, 2001. |
| [14] | W. Sand, T. Gehrke, P. Jozsa and A. Schippers, (Bio) chemistry of bacterial leaching-direct vs. indirect bioleaching, Hydrometallurgy, 59, 159-175, 2001. |
| [15] | L.A. Brickett, R.W. Hammack and H.M. Edenborn, Comparison of methods used to inhibit bacterial activity in sulfide ore bioleaching studies, Hydrometallurgy, 39, 293-305, 1995. |















 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML